Enhanced oncolytic adenoviral production by downregulation of death-domain associated protein and overexpression of precursor terminal protein
- PMID: 33441685
- PMCID: PMC7807022
- DOI: 10.1038/s41598-020-79998-1
Enhanced oncolytic adenoviral production by downregulation of death-domain associated protein and overexpression of precursor terminal protein
Abstract
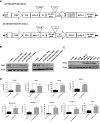
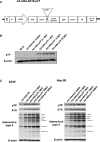
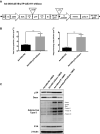
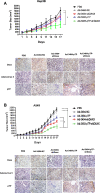
Adequate viral replication in tumor cells is the key to improving the anti-cancer effects of oncolytic adenovirus therapy. In this study, we introduced short hairpin RNAs against death-domain associated protein (Daxx), a repressor of adenoviral replication, and precursor terminal protein (pTP), an initiator of adenoviral genome replication, into adenoviral constructs to determine their contributions to viral replication. Both Daxx downregulation and pTP overexpression increased viral production in variety of human cancer cell lines, and the enhanced production of virus progeny resulted in more cell lysis in vitro, and tumor regression in vivo. We confirmed that increased virus production by Daxx silencing, or pTP overexpression, occurred using different mechanisms by analyzing levels of adenoviral protein expression and virus production. Specifically, Daxx downregulation promoted both virus replication and oncolysis in a consecutive manner by optimizing IVa2-based packaging efficiency, while pTP overexpression by increasing both infectious and total virus particles but their contribution to increased viral production may have been damaged to some extent by their another contribution to apoptosis and autophagy. Therefore, introducing both Daxx shRNA and pTP in virotherapy may be a suitable strategy to increase apoptotic tumor-cell death and to overcome poor viral replication, leading to meaningful reductions in tumor growth in vivo.
Conflict of interest statement
The authors declare no competing interests.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

