Culex quinquefasciatus carrying Wolbachia is less susceptible to entomopathogenic bacteria
- PMID: 33441735
- PMCID: PMC7806911
- DOI: 10.1038/s41598-020-80034-5
Culex quinquefasciatus carrying Wolbachia is less susceptible to entomopathogenic bacteria
Abstract
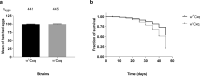
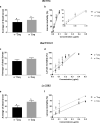
In an attempt to evaluate the susceptibility of the mosquito Culex quinquefasciatus to bacterial agents, a population naturally infected with a Wolbachia pipientis wPipSJ native strain was tested against the action of three bacterial mosquitocides, Bacillus thuringiensis subsp. israelensis, Bacillus wiedmannii biovar thuringiensis and Lysinibacillus sphaericus. Tests were carried out on mosquito larvae with and without Wolbachia (controls). Cx. quinquefasciatus naturally infected with the native wPipSJ strain proved to be more resistant to the pathogenic action of the three mosquitocidal bacterial strains. Additionally, wPipSJ was fully characterised using metagenome-assembled genomics, PCR-RFLP (PCR-Restriction Fragment Length Polymorphism) and MLST (MultiLocus Sequence Typing) analyses. This Wolbachia strain wPipSJ belongs to haplotype I, group wPip-III and supergroup B, clustering with other mosquito wPip strains, such as wPip PEL, wPip JHB, wPip Mol, and wAlbB; showing the southernmost distribution in America. The cytoplasmic incompatibility phenotype of this strain was revealed via crosses between wildtype (Wolbachia+) and antibiotic treated mosquito populations. The results of the tests with the bacterial agents suggest that Cx. quinquefasciatus naturally infected with wPipSJ is less susceptible to the pathogenic action of mosquitocidal bacterial strains when compared with the antibiotic-treated mosquito isoline, and is more susceptible to B. thuringiensis subsp. israelensis than to the other two mosquitocidal agents.
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

