Colony spreading of the gliding bacterium Flavobacterium johnsoniae in the absence of the motility adhesin SprB
- PMID: 33441737
- PMCID: PMC7807042
- DOI: 10.1038/s41598-020-79762-5
Colony spreading of the gliding bacterium Flavobacterium johnsoniae in the absence of the motility adhesin SprB
Abstract
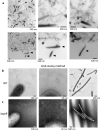
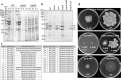
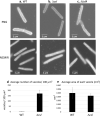
Colony spreading of Flavobacterium johnsoniae is shown to include gliding motility using the cell surface adhesin SprB, and is drastically affected by agar and glucose concentrations. Wild-type (WT) and ΔsprB mutant cells formed nonspreading colonies on soft agar, but spreading dendritic colonies on soft agar containing glucose. In the presence of glucose, an initial cell growth-dependent phase was followed by a secondary SprB-independent, gliding motility-dependent phase. The branching pattern of a ΔsprB colony was less complex than the pattern formed by the WT. Mesoscopic and microstructural information was obtained by atmospheric scanning electron microscopy (ASEM) and transmission EM, respectively. In the growth-dependent phase of WT colonies, dendritic tips spread rapidly by the movement of individual cells. In the following SprB-independent phase, leading tips were extended outwards by the movement of dynamic windmill-like rolling centers, and the lipoproteins were expressed more abundantly. Dark spots in WT cells during the growth-dependent spreading phase were not observed in the SprB-independent phase. Various mutations showed that the lipoproteins and the motility machinery were necessary for SprB-independent spreading. Overall, SprB-independent colony spreading is influenced by the lipoproteins, some of which are involved in the gliding machinery, and medium conditions, which together determine the nutrient-seeking behavior.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources

