Berberine-releasing electrospun scaffold induces osteogenic differentiation of DPSCs and accelerates bone repair
- PMID: 33441759
- PMCID: PMC7806735
- DOI: 10.1038/s41598-020-79734-9
Berberine-releasing electrospun scaffold induces osteogenic differentiation of DPSCs and accelerates bone repair
Abstract
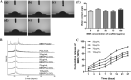
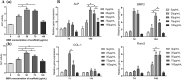
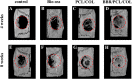
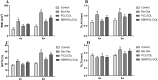
The repair of skeletal defects in maxillofacial region remains an intractable problem, the rising technology of bone tissue engineering provides a new strategy to solve it. Scaffolds, a crucial element of tissue engineering, must have favorable biocompatibility as well as osteoinductivity. In this study, we prepared berberine/polycaprolactone/collagen (BBR/PCL/COL) scaffolds with different concentrations of berberine (BBR) (25, 50, 75 and 100 μg/mL) through electrospinning. The influence of dosage on scaffold morphology, cell behavior and in vivo bone defect repair were systematically studied. The results indicated that scaffolds could release BBR stably for up to 27 days. Experiments in vitro showed that BBR/PCL/COL scaffolds had appropriate biocompatibility in the concentration of 25-75 μg/mL, and 50 and 75 μg/mL scaffolds could significantly promote osteogenic differentiation of dental pulp stem cells. Scaffold with 50 μg/mL BBR was implanted into the critical bone defect of rats to evaluate the ability of bone repair in vivo. It was found that BBR/PCL/COL scaffold performed more favorable than polycaprolactone/collagen (PCL/COL) scaffold. Overall, our study is the first to evaluate the capability of in vivo bone repair of BBR/PCL/COL electrospun scaffold. The results indicate that BBR/PCL/COL scaffold has prospective potential for tissue engineering applications in bone regeneration therapy.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

