Maternal Roux-en-Y gastric bypass surgery reduces lipid deposition and increases UCP1 expression in the brown adipose tissue of male offspring
- PMID: 33441773
- PMCID: PMC7806700
- DOI: 10.1038/s41598-020-80104-8
Maternal Roux-en-Y gastric bypass surgery reduces lipid deposition and increases UCP1 expression in the brown adipose tissue of male offspring
Abstract
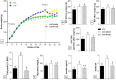
Maternal obesity induced by cafeteria diet (CAF) predisposes offspring to obesity and metabolic diseases, events that could be avoided by maternal bariatric surgery (BS). Herein we evaluated whether maternal BS is able to modulate brown adipose tissue (BAT) morphology and function in adult male rats born from obese female rats submitted to Roux-en-Y gastric bypass (RYGB). For this, adult male rat offspring were obtained from female rats that consumed standard diet (CTL), or CAF diet, and were submitted to simulated operation or RYGB. Analysis of offspring showed that, at 120 days of life, the maternal CAF diet induced adiposity and decreased the expression of mitochondrial Complex I (CI) and Complex III (CIII) in the BAT, resulting in higher accumulation of lipids than in BAT from offspring of CTL dams. Moreover, maternal RYGB increased UCP1 expression and prevented excessive deposition of lipids in the BAT of adult male offspring rats. However, maternal RYGB failed to reverse the effects of maternal diet on CI and CIII expression. Thus, maternal CAF promotes higher lipid deposition in the BAT of offspring, contributing to elevated adiposity. Maternal RYGB prevented obesity in offspring, probably by increasing the expression of UCP1.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

