Enhanced hippocampal type II theta activity AND altered theta architecture in mice lacking the Cav3.2 T-type voltage-gated calcium channel
- PMID: 33441788
- PMCID: PMC7806756
- DOI: 10.1038/s41598-020-79763-4
Enhanced hippocampal type II theta activity AND altered theta architecture in mice lacking the Cav3.2 T-type voltage-gated calcium channel
Abstract
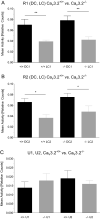
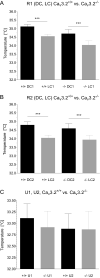
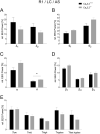
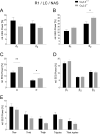
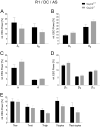
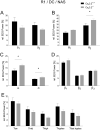
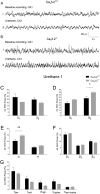
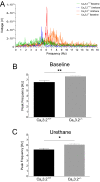
T-type Ca2+ channels are assumed to contribute to hippocampal theta oscillations. We used implantable video-EEG radiotelemetry and qPCR to unravel the role of Cav3.2 Ca2+ channels in hippocampal theta genesis. Frequency analysis of spontaneous long-term recordings in controls and Cav3.2-/- mice revealed robust increase in relative power in the theta (4-8 Hz) and theta-alpha (4-12 Hz) ranges, which was most prominent during the inactive stages of the dark cycles. Urethane injection experiments also showed enhanced type II theta activity and altered theta architecture following Cav3.2 ablation. Next, gene candidates from hippocampal transcriptome analysis of control and Cav3.2-/- mice were evaluated using qPCR. Dynein light chain Tctex-Type 1 (Dynlt1b) was significantly reduced in Cav3.2-/- mice. Furthermore, a significant reduction of GABA A receptor δ subunits and GABA B1 receptor subunits was observed in the septohippocampal GABAergic system. Our results demonstrate that ablation of Cav3.2 significantly alters type II theta activity and theta architecture. Transcriptional changes in synaptic transporter proteins and GABA receptors might be functionally linked to the electrophysiological phenotype.
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Spontaneous long-term and urethane induced hippocampal EEG power, activity and temperature data from mice lacking the Cav3.2 voltage-gated Ca2+ channel.Data Brief. 2021 Apr 1;36:107027. doi: 10.1016/j.dib.2021.107027. eCollection 2021 Jun. Data Brief. 2021. PMID: 33948455 Free PMC article.
-
Cav3 T-Type Voltage-Gated Ca2+ Channels and the Amyloidogenic Environment: Pathophysiology and Implications on Pharmacotherapy and Pharmacovigilance.Int J Mol Sci. 2022 Mar 22;23(7):3457. doi: 10.3390/ijms23073457. Int J Mol Sci. 2022. PMID: 35408817 Free PMC article. Review.
-
Suppression of Sleep Spindle Rhythmogenesis in Mice with Deletion of CaV3.2 and CaV3.3 T-type Ca(2+) Channels.Sleep. 2016 Apr 1;39(4):875-85. doi: 10.5665/sleep.5646. Sleep. 2016. PMID: 26612388 Free PMC article.
-
CACHD1 is an α2δ-Like Protein That Modulates CaV3 Voltage-Gated Calcium Channel Activity.J Neurosci. 2018 Oct 24;38(43):9186-9201. doi: 10.1523/JNEUROSCI.3572-15.2018. Epub 2018 Sep 4. J Neurosci. 2018. PMID: 30181139 Free PMC article.
-
Functional importance of T-type voltage-gated calcium channels in the cardiovascular and renal system: news from the world of knockout mice.Am J Physiol Regul Integr Comp Physiol. 2015 Feb 15;308(4):R227-37. doi: 10.1152/ajpregu.00276.2014. Epub 2014 Dec 17. Am J Physiol Regul Integr Comp Physiol. 2015. PMID: 25519728 Review.
Cited by
-
Breeding of Cav2.3 deficient mice reveals Mendelian inheritance in contrast to complex inheritance in Cav3.2 null mutant breeding.Sci Rep. 2021 Jul 7;11(1):13972. doi: 10.1038/s41598-021-93391-6. Sci Rep. 2021. PMID: 34234221 Free PMC article.
-
Maturational Stage-Dependent Contributions of the Cav3.2 T-Type Calcium Channel to Dentate Gyrus Granule Cell Excitability.eNeuro. 2025 Apr 4;12(4):ENEURO.0423-24.2025. doi: 10.1523/ENEURO.0423-24.2025. Print 2025 Apr. eNeuro. 2025. PMID: 40068874 Free PMC article.
-
The Medial Septum as a Potential Target for Treating Brain Disorders Associated With Oscillopathies.Front Neural Circuits. 2021 Jul 8;15:701080. doi: 10.3389/fncir.2021.701080. eCollection 2021. Front Neural Circuits. 2021. PMID: 34305537 Free PMC article. Review.
-
Spontaneous long-term and urethane induced hippocampal EEG power, activity and temperature data from mice lacking the Cav3.2 voltage-gated Ca2+ channel.Data Brief. 2021 Apr 1;36:107027. doi: 10.1016/j.dib.2021.107027. eCollection 2021 Jun. Data Brief. 2021. PMID: 33948455 Free PMC article.
-
Cav3 T-Type Voltage-Gated Ca2+ Channels and the Amyloidogenic Environment: Pathophysiology and Implications on Pharmacotherapy and Pharmacovigilance.Int J Mol Sci. 2022 Mar 22;23(7):3457. doi: 10.3390/ijms23073457. Int J Mol Sci. 2022. PMID: 35408817 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

