Human α-synuclein overexpression in a mouse model of Parkinson's disease leads to vascular pathology, blood brain barrier leakage and pericyte activation
- PMID: 33441868
- PMCID: PMC7806665
- DOI: 10.1038/s41598-020-80889-8
Human α-synuclein overexpression in a mouse model of Parkinson's disease leads to vascular pathology, blood brain barrier leakage and pericyte activation
Abstract
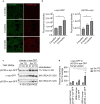
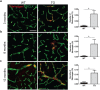
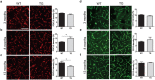
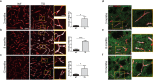
The pathological hallmark of Parkinson's disease (PD) is the formation of Lewy bodies containing aggregated alpha-synuclein (α-syn). Although PD is associated with these distinct histological changes, other pathological features such as microvascular alterations have been linked to neurodegeneration. These changes need to be investigated as they create a hostile brain microenvironment and may contribute to the development and progression of the disease. We use a human α-syn overexpression mouse model that recapitulates some of the pathological features of PD in terms of progressive aggregation of human α-syn, impaired striatal dopamine fiber density, and an age-dependent motor deficit consistent with an impaired dopamine release. We demonstrate for the first time in this model a compromised blood-brain barrier integrity and dynamic changes in vessel morphology from angiogenesis at earlier stages to vascular regression at later stages. The vascular alterations are accompanied by a pathological activation of pericytes already at an early stage without changing overall pericyte density. Our data support and further extend the occurrence of vascular pathology as an important pathophysiological aspect in PD. The model used provides a powerful tool to investigate disease-modifying factors in PD in a temporal sequence that might guide the development of new treatments.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Fearnley, J. M. & Lees, A. J. Ageing and parkinson’s disease: Substantia nigra regional selectivity. Brain114. https://academic.oup.com/brain/article-abstract/114/5/2283/399854 (1991). - PubMed
-
- Wada, K. et al. Expression levels of vascular endothelial growth factor and its receptors in Parkinson’s disease. (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

