Preliminary clinical experience applying donor-derived cell-free DNA to discern rejection in pediatric liver transplant recipients
- PMID: 33441886
- PMCID: PMC7807012
- DOI: 10.1038/s41598-020-80845-6
Preliminary clinical experience applying donor-derived cell-free DNA to discern rejection in pediatric liver transplant recipients
Abstract
Donor-derived cell-free DNA (dd-cfDNA) has been of major interest recently as a non-invasive marker of graft injury, but has not yet been extensively tested in children. From May to September in 2019, a total of 76 pediatric patients receiving a liver graft were enrolled and there were 27 patients excluded. Ultimately plasma samples and matched liver specimens from 49 patients were successfully collected whenever rejection was suspected clinically. Dd-cfDNA were analyzed and then compared to biopsy. Of these, 11 (22.4%) patients were found to have rejection by biopsy. Dd-cfDNA levels were higher among patients with rejection compared to those with no rejection. In subgroup analysis, dd-cfDNA% among patients with rejection differed from those with EBV/CMV infection and DILI patients. Similarly, observations were available concerning dd-cfDNA (cp/mL). The AUC for dd-cfDNA% and dd-cfDNA (cp/mL) were 0.878, 0.841, respectively, both of which were higher than conventional LFTs. For rejection, dd-cfDNA% ≥ 28.7% yielded a sensitivity of 72.7%, specificity 94.7% and dd-cfDNA (cp/mL) ≥ 2076 cp/mL, yielded a sensitivity of 81.8%, specificity 81.9%. Of note, the dd-cfDNA distribution was significantly different between whole liver and LLS transplantation. In the setting of pediatric LTx, dd-cfDNA appears to be a sensitive biomarker indicating the presence of rejection.International Clinical Trails Registry Platform: ChiCTR1900022406.
Conflict of interest statement
The authors declare no competing interests.
Figures
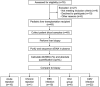

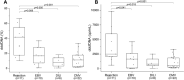
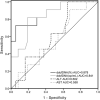

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

