Serotonin receptor 4 in the hippocampus modulates mood and anxiety
- PMID: 33441982
- PMCID: PMC8275670
- DOI: 10.1038/s41380-020-00994-y
Serotonin receptor 4 in the hippocampus modulates mood and anxiety
Abstract
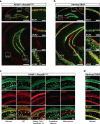
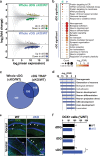
Serotonin receptor 4 (5-HT4R) plays an important role in regulating mood, anxiety, and cognition, and drugs that activate this receptor have fast-acting antidepressant (AD)-like effects in preclinical models. However, 5-HT4R is widely expressed throughout the central nervous system (CNS) and periphery, making it difficult to pinpoint the cell types and circuits underlying its effects. Therefore, we generated a Cre-dependent 5-HT4R knockout mouse line to dissect the function of 5-HT4R in specific brain regions and cell types. We show that the loss of functional 5-HT4R specifically from excitatory neurons of hippocampus led to robust AD-like behavioral responses and an elevation in baseline anxiety. 5-HT4R was necessary to maintain the proper excitability of dentate gyrus (DG) granule cells and cell type-specific molecular profiling revealed a dysregulation of genes necessary for normal neural function and plasticity in cells lacking 5-HT4R. These adaptations were accompanied by an increase in the number of immature neurons in ventral, but not dorsal, dentate gyrus, indicating a broad impact of 5-HT4R loss on the local cellular environment. This study is the first to use conditional genetic targeting to demonstrate a direct role for hippocampal 5-HT4R signaling in modulating mood and anxiety. Our findings also underscore the need for cell type-based approaches to elucidate the complex action of neuromodulatory systems on distinct neural circuits.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

