Regulation of immune responses by the airway epithelial cell landscape
- PMID: 33442032
- PMCID: PMC7804588
- DOI: 10.1038/s41577-020-00477-9
Regulation of immune responses by the airway epithelial cell landscape
Abstract
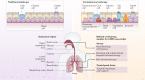
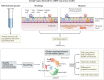
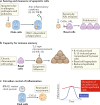
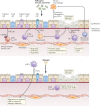
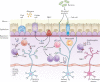
The community of cells lining our airways plays a collaborative role in the preservation of immune homeostasis in the lung and provides protection from the pathogens and pollutants in the air we breathe. In addition to its structural attributes that provide effective mucociliary clearance of the lower airspace, the airway epithelium is an immunologically active barrier surface that senses changes in the airway environment and interacts with resident and recruited immune cells. Single-cell RNA-sequencing is illuminating the cellular heterogeneity that exists in the airway wall and has identified novel cell populations with unique molecular signatures, trajectories of differentiation and diverse functions in health and disease. In this Review, we discuss how our view of the airway epithelial landscape has evolved with the advent of transcriptomic approaches to cellular phenotyping, with a focus on epithelial interactions with the local neuronal and immune systems.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Rhodin JA. The ciliated cell. Ultrastructure and function of the human tracheal mucosa. Am. Rev. Respir. Dis. 1966;93 (Suppl.):1–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

