Exceptional Regression of Malignant Pleural Mesothelioma with Pembrolizumab Monotherapy
- PMID: 33442373
- PMCID: PMC7772860
- DOI: 10.1159/000512013
Exceptional Regression of Malignant Pleural Mesothelioma with Pembrolizumab Monotherapy
Abstract
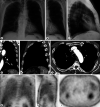
The lead author with clinical stage I malignant pleural mesothelioma, epithelioid type, highly programmed cell death ligand 1 (PD-L1) positive, and BAP1 negative, experienced a prompt and exceptionally favorable response to pembrolizumab monotherapy. After cessation of treatment due to immune-related endocrinopathies, complete metabolic response on interim PET/CT scan was achieved. Two years after initial diagnosis, unifocal tumor reactivation was addressed with successful pembrolizumab monotherapy rechallenge. Immunotherapy, typically not used as frontline treatment for malignant pleural mesothelioma, may provide an effective and durable response for some patients. Based on this single case study, epithelioid type tumors with strongly positive PD-L1 and BAP1-negative immunohistochemical markers may be well suited for treatment with immune checkpoint inhibitors such as pembrolizumab.
Keywords: Immune checkpoint inhibitor; Immune-related endocrinopathy; Malignant pleural mesothelioma regression; Pembrolizumab monotherapy; Programmed cell death ligand 1 expression; Therapeutic biomarkers.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest.
Figures

References
-
- Bronte G, Delmonte A, Burgio MA, Verlicchi A, Puccetti M, Bravaccini S, et al. Impressive clinical response to anti-PD-1 therapy in epithelioid mesothelioma with high clonal PD-L1 expression and EML4-ALK rearrangement. Lung Cancer. 2020;142:47–50. - PubMed
-
- Alley EW, Lopez J, Santoro A, Morosky A, Saraf S, Piperdi B, et al. Clinical safety and activity of pembrolizumab in patients with malignant pleural mesothelioma (KEYNOTE-028): preliminary results from a nonrandomised, open-label, phase 1b trial. Lancet Oncol. 2017;18:623–30. - PubMed
-
- Robert C, Ribas A, Hamid O, Daud A, Wolchok JD, Joshua AM, et al. Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanoma. J Clin Oncol. 2018;36((17)):1668–74. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

