LncRNA FAM83A-AS1 promotes ESCC progression by regulating miR-214/CDC25B axis
- PMID: 33442418
- PMCID: PMC7797654
- DOI: 10.7150/jca.54007
LncRNA FAM83A-AS1 promotes ESCC progression by regulating miR-214/CDC25B axis
Abstract
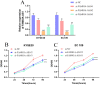
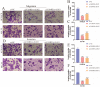
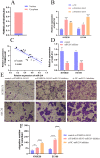
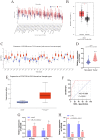
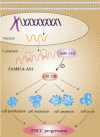
Background: Recent researches have pinpointed that long non-coding RNA (lncRNA) was tightly related to the carcinogenesis. However, the function of lncRNA in esophageal cell squamous carcinoma (ESCC) remains to be explored. In the current study, we assessed the expression pattern and the biological function of FAM83A-AS1 in ESCC. Methods: qRT-PCR was used to detect the expression of FAM83A-AS1, miR-214, and CDC25B expression in ESCC tissues and cell lines. CCK-8, transwell, apoptosis and cell cycle assays were performed to define the function of FAM83A-AS1 in ESCC cells. Furthermore, the regulation of miR-214 by FAM83A-AS1 was defined by qRT- PCR and rescue assays. In addition, the association between CDC25B, miR-214, CDC25B was confirmed by qRT-PCR. Results: Here, we discovered that FAM83A-AS1 was strongly expressed in ESCC tissues. FAM83A-AS1 abundance was associated with TNM stages and the differentiation grade of ESCC patients. The receiver operating characteristic curve (ROC) analysis indicated the high accuracy of FAM83A-AS1 in ESCC diagnosis. Functionally, inhibiting FAM83A-AS1 repressed cell proliferation, migration, and invasion in ESCC. In addition, we found that FAM83A-AS1 accelerated the cell cycle while inhibited cell apoptosis. Mechanistically, we found that FAM83A-AS1 regulated miR-214 expression, and there was a negative correlation between miR-214 and FAM83A-AS1 in ESCC. Rescue assay indicated that miR-214 could impair the suppressing effect of cell migration induced by FAM83A-AS1 depletion. Furthermore, CDC25B was a direct target of miR-214, and FAM83A-AS1 enhanced CDC25B expression while miR-214 positively CDC25B expression in ESCC. Conclusions: Collectively, we concluded that FAM83A-AS1 facilitated ESCC progression by regulating the miR-214/CDC25B axis. Our study showed FAM83A-AS1 may act as a promising target for ESCC diagnosis and therapy.
Keywords: CDC25B; ESCC; FAM83A-AS1; lncRNA; miR-214.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures



References
-
- Sun K, Zhao X, Wan J, Yang L, Chu J, Dong S. et al. The diagnostic value of long non-coding RNA MIR31HG and its role in esophageal squamous cell carcinoma. Life Science. 2018;202:124–30. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

