Responses of a hot spring cyanobacterium under ultraviolet and photosynthetically active radiation: photosynthetic performance, antioxidative enzymes, mycosporine-like amino acid profiling and its antioxidative potentials
- PMID: 33442509
- PMCID: PMC7778668
- DOI: 10.1007/s13205-020-02562-1
Responses of a hot spring cyanobacterium under ultraviolet and photosynthetically active radiation: photosynthetic performance, antioxidative enzymes, mycosporine-like amino acid profiling and its antioxidative potentials
Abstract
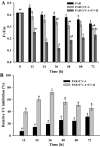
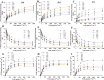
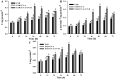
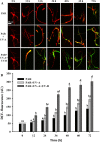
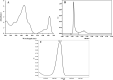
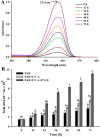
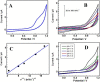
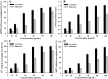
This study summarizes the response of a hot spring cyanobacterium Fischerella sp. strain HKAR-14, under simulated light conditions of ultraviolet radiation (UVR), photosynthetically active radiation (PAR), PAR + UV-A (PA) and PAR + UV-A + UV-B (PAB). Exposure to UVR caused a decline in growth and Chl a while total carotene content increased under PA and PAB. Maximum photochemical efficiency of photosystem II (F v /F m) and relative electron transport rate decreased significantly in PA and PAB exposure. Higher non-photochemical quenching and lower photochemical quenching values were observed in UVR-exposed samples as compared to the control. Levels of intracellular reactive oxygen species (ROS) increased significantly in PAB and PA. Fluorescence microscopic images showed an increase in green fluorescence, indicating the generation of ROS in UVR. The antioxidant machinery including superoxide dismutase, catalase and peroxidase showed an increase of 1.76-fold and 2.5-fold superoxide dismutase, 2.4-fold and 3.7-fold catalase, 1.83-fold and 2.5-fold peroxidase activities under PA and PAB, respectively. High-performance liquid chromatography equipped with photodiode array detector, electrospray ionization mass spectrometry, Fourier-transform infrared spectroscopy and nuclear magnetic resonance spectroscopy analyses reveal the occurrence of a single mycosporine-like amino acid, shinorine (λ max 332.3 ± 2 nm, m/z 333.1), with a retention time of 1.157 min. The electrochemical characterization of shinorine was determined by cyclic voltammetry. The shinorine molecule possesses electrochemical activity and represents diffusion-controlled process in 0.1 M (pH 7.0) phosphate buffer. An antioxidant assay of shinorine showed its efficient activity as antioxidant which increased in a dose-dependent manner.
Keywords: Antioxidant; Chlorophyll fluorescence; Cyanobacteria; Mycosporine-like amino acids; Ultraviolet radiation.
© King Abdulaziz City for Science and Technology 2021.
Conflict of interest statement
Conflict of interestAuthors declare no conflict of interest.
Figures


Similar articles
-
Impacts of ultraviolet and photosynthetically active radiations on photosynthetic efficiency and antioxidant systems of the cyanobacterium Spirulina subsalsa HKAR-19.Folia Microbiol (Praha). 2024 Aug;69(4):747-765. doi: 10.1007/s12223-023-01110-7. Epub 2023 Dec 2. Folia Microbiol (Praha). 2024. PMID: 38041744
-
Extraction, characterization and antioxidative potentials of UV-screening compound, mycosporine-like amino acids from epilithic cyanobacterium Lyngbya sp. HKAR - 15.World J Microbiol Biotechnol. 2024 Nov 6;40(12):378. doi: 10.1007/s11274-024-04184-8. World J Microbiol Biotechnol. 2024. PMID: 39503910
-
Effects of ultraviolet and photosynthetically active radiation on morphogenesis, antioxidants and photoprotective defense mechanism in a hot-spring cyanobacterium Nostoc sp. strain VKB02.Res Microbiol. 2024 Jul-Aug;175(5-6):104180. doi: 10.1016/j.resmic.2024.104180. Epub 2024 Jan 8. Res Microbiol. 2024. PMID: 38199600
-
Mycosporine-Like Amino Acids: Making the Foundation for Organic Personalised Sunscreens.Mar Drugs. 2019 Nov 12;17(11):638. doi: 10.3390/md17110638. Mar Drugs. 2019. PMID: 31726795 Free PMC article. Review.
-
Bioinspired biomolecules: Mycosporine-like amino acids and scytonemin from Lyngbya sp. with UV-protection potentialities.J Photochem Photobiol B. 2019 Dec;201:111684. doi: 10.1016/j.jphotobiol.2019.111684. Epub 2019 Nov 6. J Photochem Photobiol B. 2019. PMID: 31733505 Review.
Cited by
-
Impacts of PAR and UV radiation on diurnal photosynthesis performance, pigment composition, and antioxidant function of the hot-spring cyanobacterium Nostoc sp. strain VKB02.Arch Microbiol. 2025 May 12;207(6):144. doi: 10.1007/s00203-025-04338-8. Arch Microbiol. 2025. PMID: 40353896
-
Physiological responses of the cyanobacterium Synechocystis sp. PCC 6803 under rhythmic light variations.Photochem Photobiol Sci. 2023 Sep;22(9):2055-2069. doi: 10.1007/s43630-023-00429-x. Epub 2023 May 25. Photochem Photobiol Sci. 2023. PMID: 37227683
References
-
- Aebi H (1984) Catalase in vitro. Methods in enzymology. In: Packer L (ed) Academic Press Inc, San Diego, pp 121–126. https://doi.org/10.1016/S0076-6879(84)05016-3 - PubMed
-
- Agrawal S, Singh S, Agrawal M. Ultraviolet-B induced changes in gene expression and antioxidants in plants. Adv Bot Res. 2009;52:47–86. doi: 10.1016/S0065-2296(10)52003-2. - DOI
-
- Ahmed H, Pathak J, Rajneesh, Pandey A, Singh PR, Sinha RP (2019) Genomics and proteomics of photoprotective compound mycosporine-like amino acids in cyanobacteria. Innovations in Life Science Research. Nova Science Publishers, Hauppauge, pp 97–128
LinkOut - more resources
Full Text Sources
Other Literature Sources

