Diagnosing COVID-19 pneumonia in a pandemic setting: Lung Ultrasound versus CT (LUVCT) - a multicentre, prospective, observational study
- PMID: 33442553
- PMCID: PMC7569754
- DOI: 10.1183/23120541.00539-2020
Diagnosing COVID-19 pneumonia in a pandemic setting: Lung Ultrasound versus CT (LUVCT) - a multicentre, prospective, observational study
Abstract
Background: In this coronavirus disease 2019 (COVID-19) pandemic, fast and accurate testing is needed to profile patients at the emergency department (ED) and efficiently allocate resources. Chest imaging has been considered in COVID-19 workup, but evidence on lung ultrasound (LUS) is sparse. We therefore aimed to assess and compare the diagnostic accuracy of LUS and computed tomography (CT) in suspected COVID-19 patients.
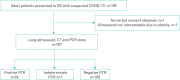
Methods: This multicentre, prospective, observational study included adult patients with suspected COVID-19 referred to internal medicine at the ED. We calculated diagnostic accuracy measures for LUS and CT using both PCR and multidisciplinary team (MDT) diagnosis as reference. We also assessed agreement between LUS and CT, and between sonographers.
Results: One hundred and eighty-seven patients were recruited between March 19 and May 4, 2020. Area under the receiver operating characteristic (AUROC) was 0.81 (95% CI 0.75-0.88) for LUS and 0.89 (95% CI 0.84-0.94) for CT. Sensitivity and specificity for LUS were 91.9% (95% CI 84.0-96.7) and 71.0% (95% CI 61.1-79.6), respectively, versus 88.4% (95% CI 79.7-94.3) and 82.0% (95% CI 73.1-89.0) for CT. Negative likelihood ratio was 0.1 (95% CI 0.06-0.24) for LUS and 0.14 (95% CI 0.08-0.3) for CT. No patient with a false negative LUS required supplemental oxygen or admission. LUS specificity increased to 80% (95% CI 69.9-87.9) compared to MDT diagnosis, with an AUROC of 0.85 (95% CI 0.79-0.91). Agreement between LUS and CT was 0.65. Interobserver agreement for LUS was good: 0.89 (95% CI 0.83-0.93).
Conclusion: LUS and CT have comparable diagnostic accuracy for COVID-19 pneumonia. LUS can safely exclude clinically relevant COVID-19 pneumonia and may aid COVID-19 diagnosis in high prevalence situations.
Copyright ©ERS 2020.
Conflict of interest statement
Conflict of interest: A.W.E. Lieveld has nothing to disclose. Conflict of interest: B. Kok has nothing to disclose. Conflict of interest: F.H. Schuit has nothing to disclose. Conflict of interest: K. Azijli has nothing to disclose. Conflict of interest: J. Heijmans has nothing to disclose. Conflict of interest: A. van Laarhoven has nothing to disclose. Conflict of interest: N.L. Assman has nothing to disclose. Conflict of interest: R.S. Kootte has nothing to disclose. Conflict of interest: T.J. Olgers has nothing to disclose. Conflict of interest: P.W.B. Nanayakkara has nothing to disclose. Conflict of interest: F.H. Bosch has nothing to disclose.
Figures
Comment in
-
In the ED, LUS and CT did not differ for sensitivity or specificity for diagnosing COVID-19 pneumonia.Ann Intern Med. 2021 May;174(5):JC57. doi: 10.7326/ACPJ202105180-057. Epub 2021 May 4. Ann Intern Med. 2021. PMID: 33939488
References
-
- https://coronavirus.jhu.edu/data/new-cases Johns Hopkins Coronavirus Resource Center. New Cases of COVID-19 in World Countries. Date last accessed: June 9, 2020. Date last updated June 9, 2020.
-
- www.cdc.gov/coronavirus/2019-ncov/php/public-health-recommendations.html Centers for Disease Control and Prevention. Coronavirus Disease 2019 (COVID-19). Date last accessed: May 21, 2020. Date last updated: May 21, 2020.
LinkOut - more resources
Full Text Sources