A Roadmap to Inform Development, Validation and Approval of Digital Mobility Outcomes: The Mobilise-D Approach
- PMID: 33442578
- PMCID: PMC7768123
- DOI: 10.1159/000512513
A Roadmap to Inform Development, Validation and Approval of Digital Mobility Outcomes: The Mobilise-D Approach
Abstract
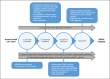
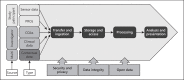
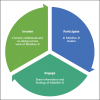
Health care has had to adapt rapidly to COVID-19, and this in turn has highlighted a pressing need for tools to facilitate remote visits and monitoring. Digital health technology, including body-worn devices, offers a solution using digital outcomes to measure and monitor disease status and provide outcomes meaningful to both patients and health care professionals. Remote monitoring of physical mobility is a prime example, because mobility is among the most advanced modalities that can be assessed digitally and remotely. Loss of mobility is also an important feature of many health conditions, providing a read-out of health as well as a target for intervention. Real-world, continuous digital measures of mobility (digital mobility outcomes or DMOs) provide an opportunity for novel insights into health care conditions complementing existing mobility measures. Accepted and approved DMOs are not yet widely available. The need for large collaborative efforts to tackle the critical steps to adoption is widely recognised. Mobilise-D is an example. It is a multidisciplinary consortium of 34 institutions from academia and industry funded through the European Innovative Medicines Initiative 2 Joint Undertaking. Members of Mobilise-D are collaborating to address the critical steps for DMOs to be adopted in clinical trials and ultimately health care. To achieve this, the consortium has developed a roadmap to inform the development, validation and approval of DMOs in Parkinson's disease, multiple sclerosis, chronic obstructive pulmonary disease and recovery from proximal femoral fracture. Here we aim to describe the proposed approach and provide a high-level view of the ongoing and planned work of the Mobilise-D consortium. Ultimately, Mobilise-D aims to stimulate widespread adoption of DMOs through the provision of device agnostic software, standards and robust validation in order to bring digital outcomes from concept to use in clinical trials and health care.
Keywords: Body-worn devices; Digital mobility outcomes; Remote Monitoring.
Copyright © 2020 by S. Karger AG, Basel.
Conflict of interest statement
L.R. receives research funding from NIHR, EU, MRC, Stroke Association, Parkinsons UK, Cure Parkinsons Trust, Dunhill Medical Trust, Health Research Council, New Zealand. She has served on Ad Board for Biogen. A.A.A. is employee of EMD Serono (a business of Merck KGaA, Darmstadt, Germany). C.B. has received speaker honoraria from Amgen, Pfizer and Nutricia. He has received fees for consultation for E. Lilly on the development of myostatin inhibitors. He has been consulting Philips Health Care/Germany, Bosch Health Care on usage of IMUs and PERS in health care. J.G.-A. reports payments for consulting and lecture fees to her institution from AstraZeneca and lecture fees from Esteeve, Chiesi and Menarini outside the submitted work.
Figures
References
-
- Onder G, Rezza G, Brusaferro S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA. 2020 May;323((18)):1775–6. - PubMed
-
- Fritz S, Lusardi M. White paper: “walking speed: the sixth vital sign”. J Geriatr Phys Ther. 2009;32((2)):46–9. - PubMed
-
- Boehme P, Hansen A, Roubenoff R, Scheeren J, Herrmann M, Mondritzki T, et al. How soon will digital endpoints become a cornerstone for future drug development? Drug Discov Today. 2019 Jan;24((1)):16–9. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

