This is a preprint.
Extracellular vimentin as a target against SARS-CoV-2 host cell invasion
- PMID: 33442680
- PMCID: PMC7805437
- DOI: 10.1101/2021.01.08.425793
Extracellular vimentin as a target against SARS-CoV-2 host cell invasion
Update in
-
Extracellular Vimentin as a Target Against SARS-CoV-2 Host Cell Invasion.Small. 2022 Feb;18(6):e2105640. doi: 10.1002/smll.202105640. Epub 2021 Dec 5. Small. 2022. PMID: 34866333 Free PMC article.
Abstract
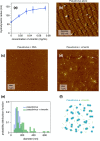
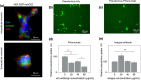
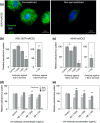
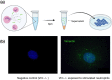
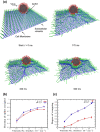
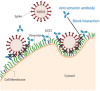
Infection of human cells by pathogens, including SARS-CoV-2, typically proceeds by cell surface binding to a crucial receptor. In the case of SARS-CoV-2, angiotensin-converting enzyme 2 (ACE2) has been identified as a necessary receptor, but not all ACE2-expressing cells are equally infected, suggesting that other extracellular factors are involved in host cell invasion by SARS-CoV-2. Vimentin is an intermediate filament protein that is increasingly recognized as being present on the extracellular surface of a subset of cell types, where it can bind to and facilitate pathogens' cellular uptake. Here, we present evidence that extracellular vimentin might act as a critical component of the SARS-CoV-2 spike protein-ACE2 complex in mediating SARS-CoV-2 cell entry. We demonstrate direct binding between vimentin and SARS-CoV-2 pseudovirus coated with the SARS-CoV-2 spike protein and show that antibodies against vimentin block in vitro SARS-CoV-2 pseudovirus infection of ACE2-expressing cells. Our results suggest new therapeutic strategies for preventing and slowing SARS-CoV-2 infection, focusing on targeting cell host surface vimentin.
Conflict of interest statement
Competing Interests: The authors declare no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
