This is a preprint.
COVID-19 virtual patient cohort reveals immune mechanisms driving disease outcomes
- PMID: 33442689
- PMCID: PMC7805446
- DOI: 10.1101/2021.01.05.425420
COVID-19 virtual patient cohort reveals immune mechanisms driving disease outcomes
Update in
-
COVID-19 virtual patient cohort suggests immune mechanisms driving disease outcomes.PLoS Pathog. 2021 Jul 14;17(7):e1009753. doi: 10.1371/journal.ppat.1009753. eCollection 2021 Jul. PLoS Pathog. 2021. PMID: 34260666 Free PMC article.
Abstract
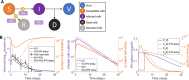
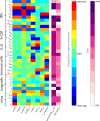
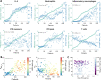
To understand the diversity of immune responses to SARS-CoV-2 and distinguish features that predispose individuals to severe COVID-19, we developed a mechanistic, within-host mathematical model and virtual patient cohort. Our results indicate that virtual patients with low production rates of infected cell derived IFN subsequently experienced highly inflammatory disease phenotypes, compared to those with early and robust IFN responses. In these in silico patients, the maximum concentration of IL-6 was also a major predictor of CD8 + T cell depletion. Our analyses predicted that individuals with severe COVID-19 also have accelerated monocyte-to-macrophage differentiation that was mediated by increased IL-6 and reduced type I IFN signalling. Together, these findings identify biomarkers driving the development of severe COVID-19 and support early interventions aimed at reducing inflammation.
Author summary: Understanding of how the immune system responds to SARS-CoV-2 infections is critical for improving diagnostic and treatment approaches. Identifying which immune mechanisms lead to divergent outcomes can be clinically difficult, and experimental models and longitudinal data are only beginning to emerge. In response, we developed a mechanistic, mathematical and computational model of the immunopathology of COVID-19 calibrated to and validated against a broad set of experimental and clinical immunological data. To study the drivers of severe COVID-19, we used our model to expand a cohort of virtual patients, each with realistic disease dynamics. Our results identify key processes that regulate the immune response to SARS-CoV-2 infection in virtual patients and suggest viable therapeutic targets, underlining the importance of a rational approach to studying novel pathogens using intra-host models.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures




References
-
- Qin C, Zhou L, Hu Z, et al. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Prepr with Lancet. 2020;February 1:1–18.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
