This is a preprint.
Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera
- PMID: 33442691
- PMCID: PMC7805448
- DOI: 10.1101/2021.01.07.425740
Neutralization of N501Y mutant SARS-CoV-2 by BNT162b2 vaccine-elicited sera
Update in
-
Neutralization of SARS-CoV-2 spike 69/70 deletion, E484K and N501Y variants by BNT162b2 vaccine-elicited sera.Nat Med. 2021 Apr;27(4):620-621. doi: 10.1038/s41591-021-01270-4. Epub 2021 Feb 8. Nat Med. 2021. PMID: 33558724
Abstract
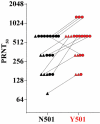
Rapidly spreading variants of SARS-CoV-2 that have arisen in the United Kingdom and South Africa share the spike N501Y substitution, which is of particular concern because it is located in the viral receptor binding site for cell entry and increases binding to the receptor (angiotensin converting enzyme 2). We generated isogenic N501 and Y501 SARS-CoV-2. Sera of 20 participants in a previously reported trial of the mRNA-based COVID-19 vaccine BNT162b2 had equivalent neutralizing titers to the N501 and Y501 viruses.
Conflict of interest statement
Competing financial interests
X.X., V.D.M., and P.-Y.S. have filed a patent on the reverse genetic system. K.A.S., M.C., D.C., and P.R.D. are employees of Pfizer and may hold stock options. X.P., J.Z., C.R.F.G., H.X., and P.-Y.S. received compensation from Pfizer to perform the neutralization assay.
Figures
References
-
- Volz E, Mishra S, Chand M et al. Report 42 - Transmission of SARS-CoV-2 Lineage B.1.1.7 in England: Insights from linking epidemiological and genetic data. https://www.imperial.ac.uk/mrc-global-infectious-disease-analysis/covid-....
-
- Tegally H, Wilkinson E, Giovanetti M et al. Emergence and rapid spread of a new severe acute respiratory syndrome-related coronavirus 2 (SARS-CoV-2) lineage with multiple spike mutations in South Afric. medRxiv 2020. 10.1101/2020.12.21.20248640 - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous