This is a preprint.
Neuropilin-1 Assists SARS-CoV-2 Infection by Stimulating the Separation of Spike Protein Domains S1 and S2
- PMID: 33442700
- PMCID: PMC7805474
- DOI: 10.1101/2021.01.06.425627
Neuropilin-1 Assists SARS-CoV-2 Infection by Stimulating the Separation of Spike Protein Domains S1 and S2
Update in
-
Neuropilin-1 assists SARS-CoV-2 infection by stimulating the separation of Spike protein S1 and S2.Biophys J. 2021 Jul 20;120(14):2828-2837. doi: 10.1016/j.bpj.2021.05.026. Epub 2021 Jun 2. Biophys J. 2021. PMID: 34087218 Free PMC article.
Abstract
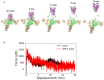
The cell surface receptor Neuropilin-1 (Nrp1) was recently identified as a host factor for SARS-CoV-2 entry. As the Spike protein of SARS-CoV-2 is cleaved into the S1 and the S2 domain by furin protease, Nrp1 binds to the newly created C-terminal RRAR amino acid sequence of the S1 domain. In this study, we model the association of a Nrp1 (a2-b1-b2) protein with the Spike protein computationally and analyze the topological constraints in the SARS-CoV-2 Spike protein for binding with Nrp1 and ACE2. Importantly, we study the exit mechanism of S2 from the S1 domain with the assistance of ACE2 as well as Nrp1 by molecular dynamics pulling simulations. In the presence of Nrp1, by binding the S1 more strongly to the host membrane, there is a high probability of S2 being pulled out, rather than S1 being stretched. Thus, Nrp1 binding could stimulate the exit of S2 from the S1 domain, which will likely increase virus infectivity as the liberated S2 domain mediates the fusion of virus and host membranes. Understanding of such a Nrp1-assisted viral infection opens the gate for the generation of protein-protein inhibitors, such as antibodies, which could attenuate the infection mechanism and protect certain cells in a future combination therapy.
Conflict of interest statement
Competing Interests The authors declare no competing interests.
Figures





References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
