This is a preprint.
The Joint Impact of COVID-19 Vaccination and Non-Pharmaceutical Interventions on Infections, Hospitalizations, and Mortality: An Agent-Based Simulation
- PMID: 33442712
- PMCID: PMC7805476
- DOI: 10.1101/2020.12.30.20248888
The Joint Impact of COVID-19 Vaccination and Non-Pharmaceutical Interventions on Infections, Hospitalizations, and Mortality: An Agent-Based Simulation
Update in
-
Association of Simulated COVID-19 Vaccination and Nonpharmaceutical Interventions With Infections, Hospitalizations, and Mortality.JAMA Netw Open. 2021 Jun 1;4(6):e2110782. doi: 10.1001/jamanetworkopen.2021.10782. JAMA Netw Open. 2021. PMID: 34061203 Free PMC article.
Abstract
Background: Vaccination against SARS-CoV-2 has the potential to significantly reduce transmission and morbidity and mortality due to COVID-19. This modeling study simulated the comparative and joint impact of COVID-19 vaccine efficacy and coverage with and without non-pharmaceutical interventions (NPIs) on total infections, hospitalizations, and deaths.
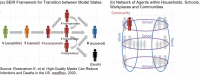
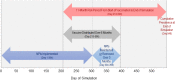
Methods: An agent-based simulation model was employed to estimate incident SARS-CoV-2 infections and COVID-19-associated hospitalizations and deaths over 18 months for the State of North Carolina, a population of roughly 10.5 million. Vaccine efficacy of 50% and 90% and vaccine coverage of 25%, 50%, and 75% (at the end of a 6-month distribution period) were evaluated. Six vaccination scenarios were simulated with NPIs (i.e., reduced mobility, school closings, face mask usage) maintained and removed during the period of vaccine distribution.
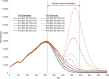
Results: In the worst-case vaccination scenario (50% efficacy and 25% coverage), 2,231,134 new SARS-CoV-2 infections occurred with NPIs removed and 799,949 infections with NPIs maintained. In contrast, in the best-case scenario (90% efficacy and 75% coverage), there were 450,575 new infections with NPIs maintained and 527,409 with NPIs removed. When NPIs were removed, lower efficacy (50%) and higher coverage (75%) reduced infection risk by a greater magnitude than higher efficacy (90%) and lower coverage (25%) compared to the worst-case scenario (absolute risk reduction 13% and 8%, respectively).
Conclusion: Simulation results suggest that premature lifting of NPIs while vaccines are distributed may result in substantial increases in infections, hospitalizations, and deaths. Furthermore, as NPIs are removed, higher vaccination coverage with less efficacious vaccines can contribute to a larger reduction in risk of SARS-CoV-2 infection compared to more efficacious vaccines at lower coverage. Our findings highlight the need for well-resourced and coordinated efforts to achieve high vaccine coverage and continued adherence to NPIs before many pre-pandemic activities can be resumed.
Conflict of interest statement
Competing Interests: The authors have no competing interests to disclose.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous