A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection
- PMID: 33443079
- PMCID: PMC7920172
- DOI: 10.1681/ASN.2020071106
A Randomized Clinical Trial of Anti-IL-6 Antibody Clazakizumab in Late Antibody-Mediated Kidney Transplant Rejection
Abstract
Background: Late antibody-mediated rejection (ABMR) is a leading cause of transplant failure. Blocking IL-6 has been proposed as a promising therapeutic strategy.
Methods: We performed a phase 2 randomized pilot trial to evaluate the safety (primary endpoint) and efficacy (secondary endpoint analysis) of the anti-IL-6 antibody clazakizumab in late ABMR. The trial included 20 kidney transplant recipients with donor-specific, antibody-positive ABMR ≥365 days post-transplantation. Patients were randomized 1:1 to receive 25 mg clazakizumab or placebo (4-weekly subcutaneous injections) for 12 weeks (part A), followed by a 40-week open-label extension (part B), during which time all participants received clazakizumab.
Results: Five (25%) patients under active treatment developed serious infectious events, and two (10%) developed diverticular disease complications, leading to trial withdrawal. Those receiving clazakizumab displayed significantly decreased donor-specific antibodies and, on prolonged treatment, modulated rejection-related gene-expression patterns. In 18 patients, allograft biopsies after 51 weeks revealed a negative molecular ABMR score in seven (38.9%), disappearance of capillary C4d deposits in five (27.8%), and resolution of morphologic ABMR activity in four (22.2%). Although proteinuria remained stable, the mean eGFR decline during part A was slower with clazakizumab compared with placebo (-0.96; 95% confidence interval [95% CI], -1.96 to 0.03 versus -2.43; 95% CI, -3.40 to -1.46 ml/min per 1.73 m2 per month, respectively, P=0.04). During part B, the slope of eGFR decline for patients who were switched from placebo to clazakizumab improved and no longer differed significantly from patients initially allocated to clazakizumab.
Conclusions: Although safety data indicate the need for careful patient selection and monitoring, our preliminary efficacy results suggest a potentially beneficial effect of clazakizumab on ABMR activity and progression.
Keywords: antibody-mediated rejection; chronic rejection; interleukin-6; monoclonal antibody; randomized controlled trial; renal transplantation.
Copyright © 2021 by the American Society of Nephrology.
Figures



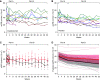
References
-
- Loupy A, Lefaucheur C: Antibody-mediated rejection of solid-organ allografts. N Engl J Med 379: 1150–1160, 2018 - PubMed
-
- Halloran PF, Venner JM, Madill-Thomsen KS, Einecke G, Parkes MD, Hidalgo LG, et al. .: Review: The transcripts associated with organ allograft rejection. Am J Transplant 18: 785–795, 2018 - PubMed
-
- Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. .: The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 18: 293–307, 2018 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

