Frequency of Biologically Defined Alzheimer Disease in Relation to Age, Sex, APOE ε4, and Cognitive Impairment
- PMID: 33443136
- PMCID: PMC8055338
- DOI: 10.1212/WNL.0000000000011416
Frequency of Biologically Defined Alzheimer Disease in Relation to Age, Sex, APOE ε4, and Cognitive Impairment
Abstract
Objective: To assess the frequency of biologically defined Alzheimer disease (AD) in relation to age, sex, APOE ε4, and clinical diagnosis in a prospective cohort study evaluated with amyloid-PET and tau-PET.
Methods: We assessed cognitively unimpaired (CU) elderly (n = 166), patients with amnestic mild cognitive impairment (n = 77), and patients with probable AD dementia (n = 62) who underwent evaluation by dementia specialists and neuropsychologists in addition to amyloid-PET with [18F]AZD4694 and tau-PET with [18F]MK6240. Individuals were grouped according to their AD biomarker profile. Positive predictive value for biologically defined AD was assessed in relation to clinical diagnosis. Frequency of AD biomarker profiles was assessed using logistic regressions with odds ratios (ORs) and 95% confidence intervals (CIs).
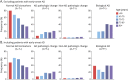
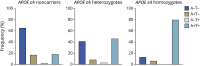
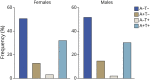
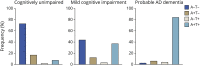
Results: The clinical diagnosis of probable AD dementia demonstrated good agreement with biologically defined AD (positive predictive value 85.2%). A total of 7.88% of CU were positive for both amyloid-PET and tau-PET. Frequency of biologically defined AD increased with age (OR 1.14; p < 0.0001) and frequency of APOE ε4 allele carriers (single ε4: OR 3.82; p < 0.0001; double ε4: OR 17.55, p < 0.0001).
Conclusion: Whereas we observed strong, but not complete, agreement between clinically defined probable AD dementia and biomarker positivity for both β-amyloid and tau, we also observed that biologically defined AD was not rare in CU elderly. Abnormal tau-PET was almost exclusively observed in individuals with abnormal amyloid-PET. Our results highlight that even in tertiary care memory clinics, detailed evaluation by dementia specialists systematically underestimates the frequency of biologically defined AD and related entities.
Classification of evidence: This study provides Class I evidence that biologically defined AD (abnormal amyloid PET and tau PET) was observed in 85.2% of people with clinically defined AD and 7.88% of CU elderly.
Copyright © 2020 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures

Comment in
-
Alzheimer Disease Spectrum: Syndrome and Etiology From Clinical and PET Imaging Perspectives.Neurology. 2021 Feb 16;96(7):299-300. doi: 10.1212/WNL.0000000000011415. Epub 2020 Dec 22. Neurology. 2021. PMID: 33361254 Free PMC article. No abstract available.
-
Author Response: Frequency of Biologically Defined Alzheimer Disease in Relation to Age, Sex, APOE ε4, and Cognitive Impairment.Neurology. 2021 Sep 21;97(12):609. doi: 10.1212/WNL.0000000000012586. Neurology. 2021. PMID: 35100130 No abstract available.
-
Reader Response: Frequency of Biologically Defined Alzheimer Disease in Relation to Age, Sex, APOE ε4, and Cognitive Impairment.Neurology. 2021 Sep 21;97(12):608. doi: 10.1212/WNL.0000000000012585. Neurology. 2021. PMID: 35100131 No abstract available.
References
-
- Ball M, Braak H, Coleman P, et al. Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. Neurobiol Aging 1997;18 (suppl 1):S1–S2. - PubMed
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer's disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer's Disease. Neurology 1984;34:939. - PubMed
-
- Lim A, Tsuang D, Kukull W, et al. Clinico-neuropathological correlation of Alzheimer's disease in a community-based case series. J Am Geriatr Soc 1999;47:564–569. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
