Tissue-specific adrenergic regulation of the L-type Ca2+ channel CaV1.2
- PMID: 33443233
- PMCID: PMC7909005
- DOI: 10.1126/scisignal.abc6438
Tissue-specific adrenergic regulation of the L-type Ca2+ channel CaV1.2
Abstract
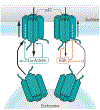
Ca2+ influx through the L-type Ca2+ channel Cav1.2 triggers each heartbeat. The fight-or-flight response induces the release of the stress response hormone norepinephrine to stimulate β-adrenergic receptors, cAMP production, and protein kinase A activity to augment Ca2+ influx through Cav1.2 and, consequently, cardiomyocyte contractility. Emerging evidence shows that Cav1.2 is regulated by different mechanisms in cardiomyocytes compared to neurons and vascular smooth muscle cells.
Copyright © 2020 The Authors, some rights reserved; exclusive licensee American Association for the Advancement of Science. No claim to original U.S. Government Works.
Figures
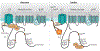
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

