A Randomized Porcine Study in Low Cardiac Output of Vasoactive and Inotropic Drug Effects on the Gastrointestinal Tract
- PMID: 33443363
- PMCID: PMC8529897
- DOI: 10.1097/SHK.0000000000001726
A Randomized Porcine Study in Low Cardiac Output of Vasoactive and Inotropic Drug Effects on the Gastrointestinal Tract
Abstract
Background: Splanchnic vasodilation by inodilators is an argument for their use in critical cardiac dysfunction. To isolate peripheral vasoactivity from inotropy, such drugs were investigated, and contrasted to vasopressors, in a fixed low cardiac output (CO) model resembling acute cardiac dysfunction effects on the gastrointestinal tract. We hypothesized that inodilators would vasodilate and preserve the aerobic metabolism in the splanchnic circulation in low CO.
Methods: In anesthetized pigs, CO was lowered to 60% of baseline by partial inferior caval vein balloon inflation. The animals were randomized to placebo (n = 8), levosimendan (24 μg kg-1 bolus, 0.2 μg kg-1 min-1, n = 7), milrinone (50 μg kg-1 bolus, 0.5 μg kg-1 min-1, n = 7), vasopressin (0.001, 0.002 and 0.006 U kg-1 min-1, 1 h each, n = 7) or norepinephrine (0.04, 0.12, and 0.36 μg kg-1 min-1, 1 h each, n = 7). Hemodynamic variables including mesenteric blood flow were collected. Systemic, mixed-venous, mesenteric-venous, and intraperitoneal metabolites were analyzed.
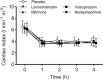
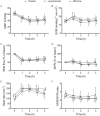
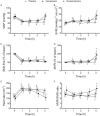
Results: Cardiac output was stable at 60% in all groups, which resulted in systemic hypotension, low superior mesenteric artery blood flow, lactic acidosis, and increased intraperitoneal concentrations of lactate. Levosimendan and milrinone did not change any circulatory variables, but levosimendan increased blood lactate concentrations. Vasopressin and norepinephrine increased systemic and mesenteric vascular resistances at the highest dose. Vasopressin increased mesenteric resistance more than systemic, and the intraperitoneal lactate concentration and lactate/pyruvate ratio.
Conclusion: Splanchnic vasodilation by levosimendan and milrinone may be negligible in low CO, thus rejecting the hypothesis. High-dose vasopressors may have side effects in the splanchnic circulation.
Copyright © 2021 by the Shock Society.
Conflict of interest statement
The authors report no conflicts of interest.
Figures
Similar articles
-
Inotropic support during experimental endotoxemic shock: part II. A comparison of levosimendan with dobutamine.Anesth Analg. 2009 Nov;109(5):1576-83. doi: 10.1213/ane.0b013e3181af40e0. Epub 2009 Aug 27. Anesth Analg. 2009. PMID: 19713252
-
Splanchnic Circulation and Intraabdominal Metabolism in Two Porcine Models of Low Cardiac Output.J Cardiovasc Transl Res. 2019 Jun;12(3):240-249. doi: 10.1007/s12265-018-9845-6. Epub 2018 Nov 19. J Cardiovasc Transl Res. 2019. PMID: 30456737 Free PMC article.
-
Inotropic support during experimental endotoxemic shock: part I. The effects of levosimendan on splanchnic perfusion.Anesth Analg. 2009 Nov;109(5):1568-75. doi: 10.1213/ane.0b013e3181af3fe3. Epub 2009 Aug 27. Anesth Analg. 2009. PMID: 19713249
-
Vasoactive drugs and the gut: is there anything new?Curr Opin Crit Care. 2006 Apr;12(2):155-9. doi: 10.1097/01.ccx.0000216584.72427.e4. Curr Opin Crit Care. 2006. PMID: 16543793 Review.
-
Inodilators in the Management of Low Cardiac Output Syndrome After Pediatric Cardiac Surgery.Curr Vasc Pharmacol. 2016;14(1):48-57. doi: 10.2174/1570161113666151014125314. Curr Vasc Pharmacol. 2016. PMID: 26463985 Review.
Cited by
-
Vasopressors and Risk of Acute Mesenteric Ischemia: A Worldwide Pharmacovigilance Analysis and Comprehensive Literature Review.Front Med (Lausanne). 2022 May 23;9:826446. doi: 10.3389/fmed.2022.826446. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35677822 Free PMC article.
-
Alternatives to norepinephrine in septic shock: Which agents and when?J Intensive Med. 2022 Jun 12;2(4):223-232. doi: 10.1016/j.jointm.2022.05.001. eCollection 2022 Oct. J Intensive Med. 2022. PMID: 36788938 Free PMC article. Review.
-
Molecular Interaction Between Vasopressin and Insulin in Regulation of Metabolism: Impact on Cardiovascular and Metabolic Diseases.Int J Mol Sci. 2024 Dec 11;25(24):13307. doi: 10.3390/ijms252413307. Int J Mol Sci. 2024. PMID: 39769071 Free PMC article. Review.
-
Whole-body mass spectrometry imaging reveals the systemic metabolic disorder and catecholamines biosynthesis alteration on heart-gut axis in heart failure rat.J Adv Res. 2025 Jul;73:411-426. doi: 10.1016/j.jare.2024.09.001. Epub 2024 Sep 11. J Adv Res. 2025. PMID: 39270978 Free PMC article.
References
-
- Hassoun HT, Kone BC, Mercer DW, Moody FG, Weisbrodt NW, Moore FA. Post-injury multiple organ failure: the role of the gut. Shock 15 (1):1–10, 2001. - PubMed
-
- Lomivorotov VV, Efremov SM, Kirov MY, Fominskiy EV, Karaskov AM. Low-cardiac-output syndrome after cardiac surgery. J Cardiothorac Vasc Anesth 31 (1):291–308, 2017. - PubMed
-
- Woolsey CA, Coopersmith CM. Vasoactive drugs and the gut: is there anything new? Curr Opin Crit Care 12 (2):155–159, 2006. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

