Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis
- PMID: 33443584
- PMCID: PMC7809610
- DOI: 10.1001/jamaoncol.2020.6973
Comparison of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis
Abstract
Importance: Multiple systemic treatments are available for metastatic castration-sensitive prostate cancer (mCSPC), with unclear comparative effectiveness and safety and widely varied costs.
Objective: To compare the effectiveness and safety determined in randomized clinical trials of systemic treatments for mCSPC.
Data sources: Bibliographic databases (MEDLINE, Embase, and Cochrane Central), regulatory documents (US Food and Drug Administration and European Medicines Agency), and trial registries (ClinicalTrials.gov and European Union clinical trials register) were searched from inception through November 5, 2019.
Study selection, data extraction, and synthesis: Eligible studies were randomized clinical trials evaluating the addition of docetaxel, abiraterone acetate, apalutamide, or enzalutamide to androgen-deprivation therapy (ADT) for treatment of mCSPC. Two investigators independently performed screening. Discrepancies were resolved through consensus. A Cochrane risk-of-bias tool was used to assess trial quality. Relative effects of competing treatments were assessed by bayesian network meta-analysis and survival models. The Preferred Reporting Items for Systematic Reviews and Meta-analyses guideline was used.
Main outcomes and measures: Overall survival, radiographic progression-free survival, and serious adverse events (SAEs).
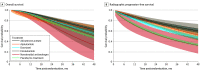
Results: Seven trials with 7287 patients comparing 6 treatments (abiraterone acetate, apalutamide, docetaxel, enzalutamide, standard nonsteroidal antiandrogen, and placebo/no treatment) were identified. Ordered from the most to the least effective determined by results of clinical trials, treatments associated with improved overall survival when added to ADT included abiraterone acetate (hazard ratio [HR], 0.61; 95% credible interval [CI], 0.54-0.70), apalutamide (HR, 0.67; 95% CI, 0.51-0.89), and docetaxel (HR, 0.79; 95% CI, 0.71-0.89); treatments associated with improved radiographic progression-free survival when added to ADT included enzalutamide (HR, 0.39; 95% CI, 0.30-0.50), apalutamide (HR, 0.48; 95% CI, 0.39-0.60), abiraterone acetate (HR, 0.51; 95% CI, 0.45-0.58), and docetaxel (HR, 0.67; 95% CI 0.60-0.74). Docetaxel was associated with substantially increased SAEs (odds ratio, 23.72; 95% CI, 13.37-45.15), abiraterone acetate with slightly increased SAEs (odds ratio, 1.42; 95% CI, 1.10-1.83), and other treatments with no significant increase in SAEs. Risk of bias was noted for 4 trials with open-label design, 3 trials with missing data, and 2 trials with potential unprespecified analyses.
Conclusions and relevance: In this network meta-analysis, as add-on treatments to ADT, abiraterone acetate and apalutamide may provide the largest overall survival benefits with relatively low SAE risks. Although enzalutamide may improve radiographic progression-free survival to the greatest extent, longer follow-up is needed to examine the overall survival benefits associated with enzalutamide.
Conflict of interest statement
Figures


Comment in
-
Considerations Regarding a Network Meta-analysis of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer.JAMA Oncol. 2021 Jul 1;7(7):1068. doi: 10.1001/jamaoncol.2021.0960. JAMA Oncol. 2021. PMID: 33983365 No abstract available.
-
Considerations Regarding a Network Meta-analysis of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer.JAMA Oncol. 2021 Jul 1;7(7):1068-1069. doi: 10.1001/jamaoncol.2021.0963. JAMA Oncol. 2021. PMID: 33983384 No abstract available.
-
Considerations Regarding a Network Meta-analysis of Systemic Treatments for Metastatic Castration-Sensitive Prostate Cancer-Reply.JAMA Oncol. 2021 Jul 1;7(7):1069-1070. doi: 10.1001/jamaoncol.2021.0966. JAMA Oncol. 2021. PMID: 33983407 No abstract available.
References
-
- US Cancer Society. Prostate at a glance. Estimated new cases, 2020. Accessed May 28, 2020. https://cancerstatisticscenter.cancer.org/#!/cancer-site/Prostate
-
- Cancer Stat Facts SEER. Prostate cancer. National Cancer Institute. Accessed May 28, 2020. https://seer.cancer.gov/statfacts/html/prost.html
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

