Role of perivascular and meningeal macrophages in outcome following experimental subarachnoid hemorrhage
- PMID: 33444089
- PMCID: PMC8327101
- DOI: 10.1177/0271678X20980296
Role of perivascular and meningeal macrophages in outcome following experimental subarachnoid hemorrhage
Abstract
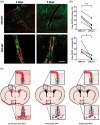
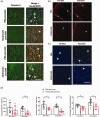
The distribution and clearance of erythrocytes after subarachnoid hemorrhage (SAH) is poorly understood. We aimed to characterize the distribution of erythrocytes after SAH and the cells involved in their clearance. To visualize erythrocyte distribution, we injected fluorescently-labelled erythrocytes into the prechiasmatic cistern of mice. 10 minutes after injection, we found labelled erythrocytes in the subarachnoid space and ventricular system, and also in the perivascular spaces surrounding large penetrating arterioles. 2 and 5 days after SAH, fluorescence was confined within leptomeningeal and perivascular cells. We identified the perivascular cells as perivascular macrophages based on their morphology, location, Iba-1 immunoreactivity and preferential uptake of FITC-dextran. We subsequently depleted meningeal and perivascular macrophages 2 days before or 3 hours after SAH with clodronate liposomes. At day 5 after SAH, we found increased blood deposition in mice treated prior to SAH, but not those treated after. Treatment post-SAH improved neurological scoring, reduced neuronal cell death and perivascular inflammation, whereas pre-treatment only reduced perivascular inflammation. Our data indicate that after SAH, erythrocytes are distributed throughout the subarachnoid space extending into the perivascular spaces of parenchymal arterioles. Furthermore, meningeal and perivascular macrophages are involved in erythrocyte uptake and play an important role in outcome after SAH.
Keywords: Perivascular macrophage; clodronate liposome; erythrocytes; inflammation; subarachnoid hemorrhage.
Conflict of interest statement
Figures





References
-
- Sandvei MS, Romundstad PlR, MüLler TommBrostrup, et al. Risk factors for aneurysmal subarachnoid hemorrhage in a prospective population study: the HUNT study in Norway. Stroke 2009; 40: 1958–1962. - PubMed
-
- Macdonald RL.Delayed neurological deterioration after subarachnoid haemorrhage. Nat Rev Neurol 2014; 10: 44–58. - PubMed
-
- Rowland MJ, Hadjipavlou G, Kelly M, et al. Delayed cerebral ischaemia after subarachnoid haemorrhage: looking beyond vasospasm. Br J Anaesth 2012; 109: 315–329. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

