Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19
- PMID: 33444289
- PMCID: PMC7934921
- DOI: 10.1172/jci.insight.143299
Circulating mitochondrial DNA is an early indicator of severe illness and mortality from COVID-19
Abstract
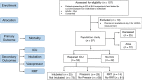
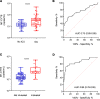
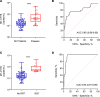
BackgroundMitochondrial DNA (MT-DNA) are intrinsically inflammatory nucleic acids released by damaged solid organs. Whether circulating cell-free MT-DNA quantitation could be used to predict the risk of poor COVID-19 outcomes remains undetermined.MethodsWe measured circulating MT-DNA levels in prospectively collected, cell-free plasma samples from 97 subjects with COVID-19 at hospital presentation. Our primary outcome was mortality. Intensive care unit (ICU) admission, intubation, vasopressor, and renal replacement therapy requirements were secondary outcomes. Multivariate regression analysis determined whether MT-DNA levels were independent of other reported COVID-19 risk factors. Receiver operating characteristic and area under the curve assessments were used to compare MT-DNA levels with established and emerging inflammatory markers of COVID-19.ResultsCirculating MT-DNA levels were highly elevated in patients who eventually died or required ICU admission, intubation, vasopressor use, or renal replacement therapy. Multivariate regression revealed that high circulating MT-DNA was an independent risk factor for these outcomes after adjusting for age, sex, and comorbidities. We also found that circulating MT-DNA levels had a similar or superior area under the curve when compared against clinically established measures of inflammation and emerging markers currently of interest as investigational targets for COVID-19 therapy.ConclusionThese results show that high circulating MT-DNA levels are a potential early indicator for poor COVID-19 outcomes.FundingWashington University Institute of Clinical Translational Sciences COVID-19 Research Program and Washington University Institute of Clinical Translational Sciences (ICTS) NIH grant UL1TR002345.
Keywords: COVID-19; Complement; Immunology; Mitochondria.
Conflict of interest statement
Figures



Update of
-
Circulating Mitochondrial DNA is an Early Indicator of Severe Illness and Mortality from COVID-19.bioRxiv [Preprint]. 2020 Jul 30:2020.07.30.227553. doi: 10.1101/2020.07.30.227553. bioRxiv. 2020. Update in: JCI Insight. 2021 Feb 22;6(4):143299. doi: 10.1172/jci.insight.143299. PMID: 32766574 Free PMC article. Updated. Preprint.
References
-
- Rilinger J, et al. A prospective, randomised, double blind placebo-controlled trial to evaluate the efficacy and safety of tocilizumab in patients with severe COVID-19 pneumonia (TOC-COVID): a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):470. doi: 10.1186/s13063-020-04447-3. - DOI - PMC - PubMed
-
- Smith K, et al. A phase 3 open-label, randomized, controlled study to evaluate the efficacy and safety of intravenously administered ravulizumab compared with best supportive care in patients with COVID-19 severe pneumonia, acute lung injury, or acute respiratory distress syndrome: a structured summary of a study protocol for a randomised controlled trial. Trials. 2020;21(1):639. doi: 10.1186/s13063-020-04548-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

