Viral load care of HIV-1 infected children and adolescents: A longitudinal study in rural Zimbabwe
- PMID: 33444325
- PMCID: PMC7808638
- DOI: 10.1371/journal.pone.0245085
Viral load care of HIV-1 infected children and adolescents: A longitudinal study in rural Zimbabwe
Abstract
Introduction: Maintaining virologic suppression of children and adolescents on ART in rural communities in sub-Saharan Africa is challenging. We explored switching drug regimens to protease inhibitor (PI) based treatment and reducing nevirapine and zidovudine use in a differentiated community service delivery model in rural Zimbabwe.
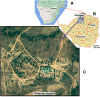
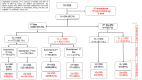
Methods: From 2016 through 2018, we followed 306 children and adolescents on ART in Hurungwe, Zimbabwe at Chidamoyo Christian Hospital, which provides compact ART regimens at 8 dispersed rural community outreach sites. Viral load testing was performed (2016) by Roche and at follow-up (2018) by a point of care viral load assay. Virologic failure was defined as viral load ≥1,000 copies/ml. A logistic regression model which included demographics, treatment regimens and caregiver's characteristics was used to assess risks for virologic failure and loss to follow-up (LTFU).
Results: At baseline in 2016, 296 of 306 children and adolescents (97%) were on first-line ART, and only 10 were receiving a PI-based regimen. The median age was 12 years (IQR 8-15) and 55% were female. Two hundred and nine (68%) had viral load suppression (<1,000 copies/ml) and 97(32%) were unsuppressed (viral load ≥1000). At follow-up in 2018, 42/306 (14%) were either transferred 23 (7%) or LTFU 17 (6%) and 2 had died. In 2018, of the 264 retained in care, 107/264 (41%), had been switched to second-line, ritonavir-boosted PI with abacavir as a new nucleotide analog reverse transcriptase inhibitor (NRTI). Overall viral load suppression increased from 68% in 2016 to 81% in 2018 (P<0.001).
Conclusion: Viral load testing, and switching to second-line, ritonavir-boosted PI with abacavir significantly increased virologic suppression among HIV-infected children and adolescents in rural Zimbabwe.
Conflict of interest statement
The authors have read the journal’s policy and the authors of this manuscript have the following competing interests: DI and MB are paid employees of Gilead Sciences Inc. There are no patents, products in development or marketed products to declare. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
References
-
- Hayes Richard, Floyd Sian, Schaap Ab, Shanaube Kwame, Bock Peter, Sabapathy Kalpana, et al. A universal testing and treatment intervention to improve HIV control: One-year results from intervention communities in Zambia in the HPTN 071 (PopART) cluster-randomised trial [Internet]. [cited 2019. September 23]. Available from: https://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1... - PMC - PubMed
-
- Mark D, Armstrong A, Andrade C, Penazzato M, Hatane L, Taing L, et al. HIV treatment and care services for adolescents: a situational analysis of 218 facilities in 23 sub-Saharan African countries. Journal of the International AIDS Society. 2017;20(S3):21591 10.7448/IAS.20.4.21591 - DOI - PMC - PubMed
-
- Mutanga JN, Mutembo S, Ezeamama AE, Song X, Fubisha RC, Mutesu-Kapembwa K, et al. Long-term survival outcomes of HIV infected children receiving antiretroviral therapy: an observational study from Zambia (2003–2015). BMC Public Health. 2019. January 28;19(1):115 10.1186/s12889-019-6444-7 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

