Polygenic risk scores in cardiovascular risk prediction: A cohort study and modelling analyses
- PMID: 33444330
- PMCID: PMC7808664
- DOI: 10.1371/journal.pmed.1003498
Polygenic risk scores in cardiovascular risk prediction: A cohort study and modelling analyses
Abstract
Background: Polygenic risk scores (PRSs) can stratify populations into cardiovascular disease (CVD) risk groups. We aimed to quantify the potential advantage of adding information on PRSs to conventional risk factors in the primary prevention of CVD.
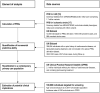
Methods and findings: Using data from UK Biobank on 306,654 individuals without a history of CVD and not on lipid-lowering treatments (mean age [SD]: 56.0 [8.0] years; females: 57%; median follow-up: 8.1 years), we calculated measures of risk discrimination and reclassification upon addition of PRSs to risk factors in a conventional risk prediction model (i.e., age, sex, systolic blood pressure, smoking status, history of diabetes, and total and high-density lipoprotein cholesterol). We then modelled the implications of initiating guideline-recommended statin therapy in a primary care setting using incidence rates from 2.1 million individuals from the Clinical Practice Research Datalink. The C-index, a measure of risk discrimination, was 0.710 (95% CI 0.703-0.717) for a CVD prediction model containing conventional risk predictors alone. Addition of information on PRSs increased the C-index by 0.012 (95% CI 0.009-0.015), and resulted in continuous net reclassification improvements of about 10% and 12% in cases and non-cases, respectively. If a PRS were assessed in the entire UK primary care population aged 40-75 years, assuming that statin therapy would be initiated in accordance with the UK National Institute for Health and Care Excellence guidelines (i.e., for persons with a predicted risk of ≥10% and for those with certain other risk factors, such as diabetes, irrespective of their 10-year predicted risk), then it could help prevent 1 additional CVD event for approximately every 5,750 individuals screened. By contrast, targeted assessment only among people at intermediate (i.e., 5% to <10%) 10-year CVD risk could help prevent 1 additional CVD event for approximately every 340 individuals screened. Such a targeted strategy could help prevent 7% more CVD events than conventional risk prediction alone. Potential gains afforded by assessment of PRSs on top of conventional risk factors would be about 1.5-fold greater than those provided by assessment of C-reactive protein, a plasma biomarker included in some risk prediction guidelines. Potential limitations of this study include its restriction to European ancestry participants and a lack of health economic evaluation.
Conclusions: Our results suggest that addition of PRSs to conventional risk factors can modestly enhance prediction of first-onset CVD and could translate into population health benefits if used at scale.
Conflict of interest statement
I have read the journal’s policy and the authors of this manuscript have the following competing interests: SK is funded by grants to institution from: British Heart Foundation, UK Medical Research Council, UK National Institute of Health Research, Cambridge Biomedical Research Centre. SB is a paid statistical reviewer for PLOS Medicine. ASB received grants outside of this work from AstraZeneca, Biogen, Bioverativ, Merck, Novartis and Sanofi, as well as personal fees from Novartis. JD serves on the International Cardiovascular and Metabolic Advisory Board for Novartis (since 2010), the Steering Committee of UK Biobank (since 2011), the MRC International Advisory Group (ING) member, London (since 2013), the MRC High Throughput Science ‘Omics Panel Member, London (since 2013), the Scientific Advisory Committee for Sanofi (since 2013), the International Cardiovascular and Metabolism Research and Development Portfolio Committee for Novartis and the Astra Zeneca Genomics Advisory Board (2018).
Figures




References
-
- UK Department of Health and Social Care. Advancing our health: prevention in the 2020s. London: UK Department of Health and Social Care; 2019 [cited 2020 Dec 18]. https://www.gov.uk/government/consultations/advancing-our-health-prevent....
Publication types
MeSH terms
Substances
Grants and funding
- MR/L003120/1/MRC_/Medical Research Council/United Kingdom
- MC_QA137853/MRC_/Medical Research Council/United Kingdom
- RG/18/13/33946/BHF_/British Heart Foundation/United Kingdom
- MC_UU_00002/7/MRC_/Medical Research Council/United Kingdom
- SP/16/4/32697/BHF_/British Heart Foundation/United Kingdom
- CH/12/2/29428/BHF_/British Heart Foundation/United Kingdom
- 204623/Z/16/Z/WT_/Wellcome Trust/United Kingdom
- MC_PC_17228/MRC_/Medical Research Council/United Kingdom
- CSO_/Chief Scientist Office/United Kingdom
- BTRU-2014-10024/DH_/Department of Health/United Kingdom
- SP/18/3/33801/BHF_/British Heart Foundation/United Kingdom
- RG/13/13/30194/BHF_/British Heart Foundation/United Kingdom
- MR/K014811/1/MRC_/Medical Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

