A novel mode of control of nickel uptake by a multifunctional metallochaperone
- PMID: 33444370
- PMCID: PMC7840056
- DOI: 10.1371/journal.ppat.1009193
A novel mode of control of nickel uptake by a multifunctional metallochaperone
Abstract
Cellular metal homeostasis is a critical process for all organisms, requiring tight regulation. In the major pathogen Helicobacter pylori, the acquisition of nickel is an essential virulence determinant as this metal is a cofactor for the acid-resistance enzyme, urease. Nickel uptake relies on the NixA permease and the NiuBDE ABC transporter. Till now, bacterial metal transporters were reported to be controlled at their transcriptional level. Here we uncovered post-translational regulation of the essential Niu transporter in H. pylori. Indeed, we demonstrate that SlyD, a protein combining peptidyl-prolyl isomerase (PPIase), chaperone, and metal-binding properties, is required for the activity of the Niu transporter. Using two-hybrid assays, we found that SlyD directly interacts with the NiuD permease subunit and identified a motif critical for this contact. Mutants of the different SlyD functional domains were constructed and used to perform in vitro PPIase activity assays and four different in vivo tests measuring nickel intracellular accumulation or transport in H. pylori. In vitro, SlyD PPIase activity is down-regulated by nickel, independently of its C-terminal region reported to bind metals. In vivo, a role of SlyD PPIase function was only revealed upon exposure to high nickel concentrations. Most importantly, the IF chaperone domain of SlyD was shown to be mandatory for Niu activation under all in vivo conditions. These data suggest that SlyD is required for the active functional conformation of the Niu permease and regulates its activity through a novel mechanism implying direct protein interaction, thereby acting as a gatekeeper of nickel uptake. Finally, in agreement with a central role of SlyD, this protein is essential for the colonization of the mouse model by H. pylori.
Conflict of interest statement
The authors have declared that no competing interests exist. Author Deborah Zamble was unable to confirm their authorship contributions. On their behalf, the corresponding author has reported their contributions to the best of their knowledge.
Figures
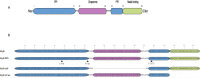

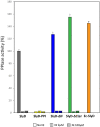
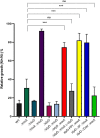


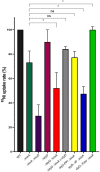


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

