Predicting in-hospital mortality from Coronavirus Disease 2019: A simple validated app for clinical use
- PMID: 33444411
- PMCID: PMC7808616
- DOI: 10.1371/journal.pone.0245281
Predicting in-hospital mortality from Coronavirus Disease 2019: A simple validated app for clinical use
Abstract
Backgrounds: Validated tools for predicting individual in-hospital mortality of COVID-19 are lacking. We aimed to develop and to validate a simple clinical prediction rule for early identification of in-hospital mortality of patients with COVID-19.
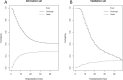
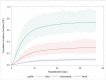
Methods and findings: We enrolled 2191 consecutive hospitalized patients with COVID-19 from three Italian dedicated units (derivation cohort: 1810 consecutive patients from Bergamo and Pavia units; validation cohort: 381 consecutive patients from Rome unit). The outcome was in-hospital mortality. Fine and Gray competing risks multivariate model (with discharge as a competing event) was used to develop a prediction rule for in-hospital mortality. Discrimination and calibration were assessed by the area under the receiver operating characteristic curve (AUC) and by Brier score in both the derivation and validation cohorts. Seven variables were independent risk factors for in-hospital mortality: age (Hazard Ratio [HR] 1.08, 95% Confidence Interval [CI] 1.07-1.09), male sex (HR 1.62, 95%CI 1.30-2.00), duration of symptoms before hospital admission <10 days (HR 1.72, 95%CI 1.39-2.12), diabetes (HR 1.21, 95%CI 1.02-1.45), coronary heart disease (HR 1.40 95% CI 1.09-1.80), chronic liver disease (HR 1.78, 95%CI 1.16-2.72), and lactate dehydrogenase levels at admission (HR 1.0003, 95%CI 1.0002-1.0005). The AUC was 0.822 (95%CI 0.722-0.922) in the derivation cohort and 0.820 (95%CI 0.724-0.920) in the validation cohort with good calibration. The prediction rule is freely available as a web-app (COVID-CALC: https://sites.google.com/community.unipa.it/covid-19riskpredictions/c19-rp).
Conclusions: A validated simple clinical prediction rule can promptly and accurately assess the risk for in-hospital mortality, improving triage and the management of patients with COVID-19.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Coronavirus disease (COVID-19) technical guidance: Laboratory testing for 2019-nCoV in humans. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technica...
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

