Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina
- PMID: 33444413
- PMCID: PMC7808630
- DOI: 10.1371/journal.ppat.1009161
Emergency response for evaluating SARS-CoV-2 immune status, seroprevalence and convalescent plasma in Argentina
Abstract
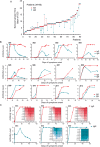
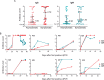
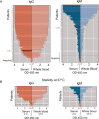
We report the emergency development and application of a robust serologic test to evaluate acute and convalescent antibody responses to SARS-CoV-2 in Argentina. The assays, COVIDAR IgG and IgM, which were produced and provided for free to health authorities, private and public health institutions and nursing homes, use a combination of a trimer stabilized spike protein and the receptor binding domain (RBD) in a single enzyme-linked immunosorbent assay (ELISA) plate. Over half million tests have already been distributed to detect and quantify antibodies for multiple purposes, including assessment of immune responses in hospitalized patients and large seroprevalence studies in neighborhoods, slums and health care workers, which resulted in a powerful tool for asymptomatic detection and policy making in the country. Analysis of antibody levels and longitudinal studies of symptomatic and asymptomatic SARS-CoV-2 infections in over one thousand patient samples provided insightful information about IgM and IgG seroconversion time and kinetics, and IgM waning profiles. At least 35% of patients showed seroconversion within 7 days, and 95% within 45 days of symptoms onset, with simultaneous or close sequential IgM and IgG detection. Longitudinal studies of asymptomatic cases showed a wide range of antibody responses with median levels below those observed in symptomatic patients. Regarding convalescent plasma applications, a protocol was standardized for the assessment of end point IgG antibody titers with COVIDAR with more than 500 plasma donors. The protocol showed a positive correlation with neutralizing antibody titers, and was used for clinical trials and therapies across the country. Using this protocol, about 80% of convalescent donor plasmas were potentially suitable for therapies. Here, we demonstrate the importance of providing a robust and specific serologic assay for generating new information about antibody kinetics in infected individuals and mitigation policies to cope with pandemic needs.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Wajnberg A, Mansour M, Leven E, Bouvier NM, Patel G, Firpo A, et al. Humoral immune response and prolonged PCR positivity in a cohort of 1343 SARS-CoV 2 patients in the New York City region 2. medRxiv. 2020; 2020.04.30.20085613. 10.1101/2020.04.30.20085613 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

