Population pharmacokinetics of clofarabine for allogeneic hematopoietic cell transplantation in paediatric patients
- PMID: 33444472
- PMCID: PMC8359279
- DOI: 10.1111/bcp.14738
Population pharmacokinetics of clofarabine for allogeneic hematopoietic cell transplantation in paediatric patients
Abstract
Aims: Clofarabine has recently been evaluated as part of the conditioning regimen for allogeneic hematopoietic stem cell transplantation (HCT) in children. Pharmacokinetic (PK) exposure of different agents commonly used in conditioning regimens is strongly related to HCT outcome. Consequently, the PK of clofarabine may be important for outcome. This report describes the population PK of clofarabine in paediatric patients and one adult.
Methods: From 80 paediatric (0.5-18 years) and 1 adult patient (37 years), 805 plasma concentrations were included in pharmacokinetic analyses using nonlinear mixed effects modelling.
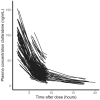
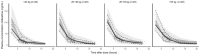
Results: A two-compartment model adequately described the PK of clofarabine. Body weight and estimated glomerular filtration rate (eGFR) were included as covariates. Clearance was differentiated into nonrenal and renal clearance (approximately 55% of total clearance), resulting in population estimates of 24.0 L/h (95% confidence interval [CI] 13.7-34.4) and 29.8 L/h (95% CI 23.9-36.1) for a patient of 70 kg with normal renal function, respectively. Unexplained interindividual variability in clearance was 17.8% (95% CI 14.6-22.4). A high variability in exposure was observed (range area under the curveT0-inf 1.8-6.0 mg/L*h) after body surface area (BSA) based dosing. Interestingly, children with low body weight had a lower exposure than children with a higher body weight, which indicates that the currently practised BSA-based dosing is not adequate for clofarabine.
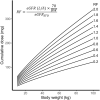
Conclusion: A clofarabine dosing algorithm based on this PK model, using body weight and eGFR, results in a more predictable exposure than BSA-based dosing. However, the exact target exposure needs to be further investigated.
Keywords: allogeneic hematopoietic cell transplantation; clinical pharmacology; clofarabine; paediatrics; pharmacokinetics.
© 2021 The Authors. British Journal of Clinical Pharmacology published by John Wiley & Sons Ltd on behalf of British Pharmacological Society.
Conflict of interest statement
There are no conflicts of interest to declare.
Figures

References
-
- Genzyme Europe BV . Summary of Product Characteristics: Evoltra. 2016. https://www.ema.europa.eu/en/documents/product-information/evoltra-epar-.... Accessed January 18, 2021.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

