Comprehensive in vivo secondary structure of the SARS-CoV-2 genome reveals novel regulatory motifs and mechanisms
- PMID: 33444546
- PMCID: PMC7775661
- DOI: 10.1016/j.molcel.2020.12.041
Comprehensive in vivo secondary structure of the SARS-CoV-2 genome reveals novel regulatory motifs and mechanisms
Abstract
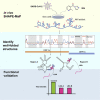
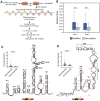
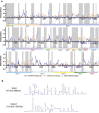
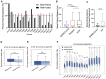
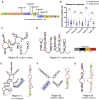
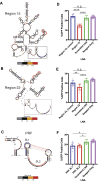
Severe-acute-respiratory-syndrome-related coronavirus 2 (SARS-CoV-2) is the positive-sense RNA virus that causes coronavirus disease 2019 (COVID-19). The genome of SARS-CoV-2 is unique among viral RNAs in its vast potential to form RNA structures, yet as much as 97% of its 30 kilobases have not been structurally explored. Here, we apply a novel long amplicon strategy to determine the secondary structure of the SARS-CoV-2 RNA genome at single-nucleotide resolution in infected cells. Our in-depth structural analysis reveals networks of well-folded RNA structures throughout Orf1ab and reveals aspects of SARS-CoV-2 genome architecture that distinguish it from other RNA viruses. Evolutionary analysis shows that several features of the SARS-CoV-2 genomic structure are conserved across β-coronaviruses, and we pinpoint regions of well-folded RNA structure that merit downstream functional analysis. The native, secondary structure of SARS-CoV-2 presented here is a roadmap that will facilitate focused studies on the viral life cycle, facilitate primer design, and guide the identification of RNA drug targets against COVID-19.
Keywords: RNA genome; RNA motif; RNA secondary structure; RNA structure; RNA virus; SHAPE-MaP; chemical probing; coronavirus; locked nucleic acids; riboregulation.
Copyright © 2020 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests A patent application on MarathonRT has been filed by Yale University.
Figures
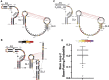

Update of
-
Comprehensive in-vivo secondary structure of the SARS-CoV-2 genome reveals novel regulatory motifs and mechanisms.bioRxiv [Preprint]. 2020 Jul 10:2020.07.10.197079. doi: 10.1101/2020.07.10.197079. bioRxiv. 2020. Update in: Mol Cell. 2021 Feb 4;81(3):584-598.e5. doi: 10.1016/j.molcel.2020.12.041. PMID: 32676598 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

