Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system
- PMID: 33444702
- PMCID: PMC7801822
- DOI: 10.1016/j.antiviral.2021.105015
Inhibition of coronavirus infection by a synthetic STING agonist in primary human airway system
Abstract
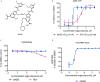
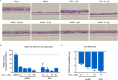
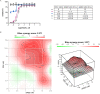
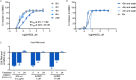
The newly emerged severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2) coronavirus initiated a pneumonia outbreak (COVID-19) that rapidly spread worldwide and quickly became a public health emergency of international concern; However to date, except Remdesivir, there are no clinically approved specific or effective medicines to prevent or treat COVID-19. Therefore, the development of novel treatments against coronavirus infections caused by the current SARS-CoV-2 virus, as well as other highly pathogenic human coronaviruses, represents an urgent unmet need. Stimulator of interferon genes (STING) plays a central role in host defense mechanisms against microbial infections. STING activation leads to the induction of both type I interferon and autophagy responses, which elicit strong inhibitory effect against the infections caused by a broad range of microbial pathogens. However, whether STING activation can impact infections from SARS-CoV-2 or other coronaviruses remains largely unknown. In this study, we investigated the anti-coronavirus activity triggered by STING activation. We discovered that dimeric amidobenzimidazole (diABZI), a synthetic small molecule STING receptor agonist, showed potent anti-coronavirus activity against both the common cold human coronavirus 229E (HCoV-229E) and SARS-CoV-2 in cell culture systems. In addition, we demonstrated that the antiviral activity of diABZI was dependent on the interferon pathway in HCoV-229E infected normal human fibroblast lung cells (MRC-5) and reconstituted primary human airway air-liquid interface (ALI) cultures. Furthermore, low-dose of diABZI treatment at 0.1 μM effectively reduced the SARS-CoV-2 viral load at the epithelial apical surface and prevented epithelial damage in the reconstituted primary human bronchial airway epithelial ALI system. Our findings have thus revealed the therapeutic potential of STING agonists, such as diABZI, as treatments for SARS-CoV-2 and other human coronavirus infections.
Keywords: Coronavirus; HCoV-229E; SARS-CoV-2; STING agonist; diABZI.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
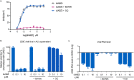
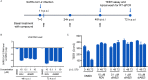
Comment in
-
A STING in the tail for SARS-CoV-2.Nat Immunol. 2021 Jul;22(7):800. doi: 10.1038/s41590-021-00971-9. Nat Immunol. 2021. PMID: 34183855 No abstract available.
Similar articles
-
A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection.Sci Immunol. 2021 May 18;6(59):eabi9002. doi: 10.1126/sciimmunol.abi9002. Sci Immunol. 2021. PMID: 34010139 Free PMC article.
-
STING Agonist Induced Innate Immune Responses Drive Anti-Respiratory Virus Activity In Vitro with Limited Antiviral Efficacy In Vivo.ACS Infect Dis. 2024 Sep 13;10(9):3392-3407. doi: 10.1021/acsinfecdis.4c00504. Epub 2024 Aug 29. ACS Infect Dis. 2024. PMID: 39207884 Free PMC article.
-
In vitro virucidal activity of Echinaforce®, an Echinacea purpurea preparation, against coronaviruses, including common cold coronavirus 229E and SARS-CoV-2.Virol J. 2020 Sep 9;17(1):136. doi: 10.1186/s12985-020-01401-2. Virol J. 2020. PMID: 32907596 Free PMC article.
-
Remdesivir against COVID-19 and Other Viral Diseases.Clin Microbiol Rev. 2020 Oct 14;34(1):e00162-20. doi: 10.1128/CMR.00162-20. Print 2020 Dec 16. Clin Microbiol Rev. 2020. PMID: 33055231 Free PMC article. Review.
-
Current status of antivirals and druggable targets of SARS CoV-2 and other human pathogenic coronaviruses.Drug Resist Updat. 2020 Dec;53:100721. doi: 10.1016/j.drup.2020.100721. Epub 2020 Aug 26. Drug Resist Updat. 2020. PMID: 33132205 Free PMC article. Review.
Cited by
-
The battle between the innate immune cGAS-STING signaling pathway and human herpesvirus infection.Front Immunol. 2023 Aug 2;14:1235590. doi: 10.3389/fimmu.2023.1235590. eCollection 2023. Front Immunol. 2023. PMID: 37600809 Free PMC article. Review.
-
K63 ubiquitination in immune signaling.Trends Immunol. 2022 Feb;43(2):148-162. doi: 10.1016/j.it.2021.12.005. Epub 2022 Jan 13. Trends Immunol. 2022. PMID: 35033428 Free PMC article. Review.
-
A diamidobenzimidazole STING agonist protects against SARS-CoV-2 infection.Sci Immunol. 2021 May 18;6(59):eabi9002. doi: 10.1126/sciimmunol.abi9002. Sci Immunol. 2021. PMID: 34010139 Free PMC article.
-
A STING agonist preconditions against ischaemic stroke via an adaptive antiviral Type 1 interferon response.Brain Commun. 2022 May 24;4(3):fcac133. doi: 10.1093/braincomms/fcac133. eCollection 2022. Brain Commun. 2022. PMID: 35694149 Free PMC article.
-
Interplay between RNA viruses and cGAS/STING axis in innate immunity.Front Cell Infect Microbiol. 2023 Apr 3;13:1172739. doi: 10.3389/fcimb.2023.1172739. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37077526 Free PMC article. Review.
References
-
- Beigel J.H., Tomashek K.M., Dodd L.E., Mehta A.K., Zingman B.S., Kalil A.C., Hohmann E., Chu H.Y., Luetkemeyer A., Kline S., Lopez de Castilla D., Finberg R.W., Dierberg K., Tapson V., Hsieh L., Patterson T.F., Paredes R., Sweeney D.A., Short W.R., Touloumi G., Lye D.C., Ohmagari N., Oh M.D., Ruiz-Palacios G.M., Benfield T., Fatkenheuer G., Kortepeter M.G., Atmar R.L., Creech C.B., Lundgren J., Babiker A.G., Pett S., Neaton J.D., Burgess T.H., Bonnett T., Green M., Makowski M., Osinusi A., Nayak S., Lane H.C., Members A.-S.G. Remdesivir for the treatment of Covid-19 - final report. N. Engl. J. Med. 2020 - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

