A glycopyrronium bromide 1% cream for topical treatment of primary axillary hyperhidrosis: efficacy and safety results from a phase IIIa randomized controlled trial
- PMID: 33445205
- PMCID: PMC8451866
- DOI: 10.1111/bjd.19810
A glycopyrronium bromide 1% cream for topical treatment of primary axillary hyperhidrosis: efficacy and safety results from a phase IIIa randomized controlled trial
Abstract
Background: Effective topical treatment options for patients with primary axillary hyperhidrosis (PAHH) are limited. A phase I trial showed promising results regarding the efficacy and safety of a topical cream containing glycopyrronium bromide (GPB).
Objectives: To assess the efficacy, safety and tolerability of a 4-week topical treatment of GPB 1% cream in patients with PAHH vs. placebo.
Methods: In total, 171 patients (84 receiving placebo; 87 receiving GPB 1%) with PAHH were included in the 4-week, multicentre, randomized, double-blind, placebo-controlled phase IIIa part of the pivotal study. Sweat production was measured by gravimetry. Patients rated the impact of disease with the Hyperhidrosis Disease Severity Scale (HDSS) and Hyperhidrosis Quality of Life Index (HidroQoL© ).
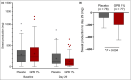
Results: Absolute change in sweat production from baseline to day 29 in logarithmic values was significantly larger in the GPB 1% group compared with the placebo group (P = 0·004). The improvement in HidroQoL exceeded the minimal clinically important difference of 4. The proportion of responders was twofold higher for sweat reduction (-197·08 mg GPB 1% vs. -83·49 mg placebo), HDSS (23% GPB 1% vs. 12% placebo) and HidroQoL (60% GPB 1% vs. 26% placebo). Treatment was safe: most treatment-emergent adverse effects were mild or moderate, and transient. Local tolerability was very good, with 9% of patients having only mild or moderate application-site reactions. The most reported adverse drug reaction was dry mouth (16%), an expected anticholinergic effect of the treatment.
Conclusions: GPB 1% cream may provide an effective new treatment option exhibiting a good safety profile for patients with PAHH. The long-term open-label part (phase IIIb) is ongoing.
© 2021 Dr August Wolff GmbH und Co KG Arzneimittel. British Journal of Dermatology published by John Wiley & Sons Ltd on behalf of British Association of Dermatologists.
Figures
Comment in
-
Pharmacokinetic profile data of glycopyrronium bromide 1% cream beyond 2 weeks are important.Br J Dermatol. 2021 Aug;185(2):467-468. doi: 10.1111/bjd.20088. Epub 2021 May 17. Br J Dermatol. 2021. PMID: 33792896 No abstract available.
-
Pharmacokinetic profile data of glycopyrronium bromide 1% cream beyond 2 weeks are important: reply from the authors.Br J Dermatol. 2021 Aug;185(2):468-469. doi: 10.1111/bjd.20095. Epub 2021 Jun 1. Br J Dermatol. 2021. PMID: 33792915 No abstract available.
References
-
- Connolly M, de Berker D. Management of primary hyperhidrosis. Am J Clin Dermatol 2003; 4:681–97. - PubMed
-
- Rzany B, Hund M. Fokale Hyperhidrose. Hautarzt 2003; 54:767–80. - PubMed
-
- Augustin M, Radtke MA, Herberger Ket al. Prevalence and disease burden of hyperhidrosis in the adult population. Dermatology 2013; 227:10–13. - PubMed
-
- Fujimoto T, Kawahara K, Yokozeki H. Epidemiological study and considerations of primary focal hyperhidrosis in Japan: from questionnaire analysis. J Dermatol 2013; 40:886–90. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

