Catalytic Conversion of Glucose into Levulinic Acid Using 2-Phenyl-2-Imidazoline Based Ionic Liquid Catalyst
- PMID: 33445440
- PMCID: PMC7827230
- DOI: 10.3390/molecules26020348
Catalytic Conversion of Glucose into Levulinic Acid Using 2-Phenyl-2-Imidazoline Based Ionic Liquid Catalyst
Abstract
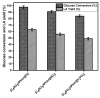
Levulinic acid (LA) is an industrially important product that can be catalytically valorized into important value-added chemicals. In this study, hydrothermal conversion of glucose into levulinic acid was attempted using Brønsted acidic ionic liquid catalyst synthesized using 2-phenyl-2-imidazoline, and 2-phenyl-2-imidazoline-based ionic liquid catalyst used in this study was synthesized in the laboratory using different anions (NO3, H2PO4, and Cl) and characterized using 1H NMR, TGA, and FT-IR spectroscopic techniques. The activity trend of the Brønsted acidic ionic liquid catalysts synthesized in the laboratory was found in the following order: [C4SO3HPhim][Cl] > [C4SO3HPhim][NO3] > [C4SO3HPhim][H2PO4]. A maximum 63% yield of the levulinic acid was obtained with 98% glucose conversion at 180 °C and 3 h reaction time using [C4SO3HPhim][Cl] ionic liquid catalyst. The effect of different reaction conditions such as reaction time, temperature, ionic liquid catalyst structures, catalyst amount, and solvents on the LA yield were investigated. Reusability of [C4SO3HPhim][Cl] catalyst up to four cycles was observed. This study demonstrates the potential of the 2-phenyl-2-imidazoline-based ionic liquid for the conversion of glucose into the important platform chemical levulinic acid.
Keywords: biomass; hydrothermal conversion; ionic liquid catalyst; levulinic acid.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






Similar articles
-
Efficient conversion of lignocellulosic biomass to levulinic acid using acidic ionic liquids.Carbohydr Polym. 2018 Feb 1;181:208-214. doi: 10.1016/j.carbpol.2017.10.064. Epub 2017 Oct 20. Carbohydr Polym. 2018. PMID: 29253965
-
Optimization of levulinic acid from lignocellulosic biomass using a new hybrid catalyst.Bioresour Technol. 2012 Jul;116:58-65. doi: 10.1016/j.biortech.2012.03.097. Epub 2012 Apr 10. Bioresour Technol. 2012. PMID: 22609656
-
The performance of 1,3-dipropyl-2-(2-propoxyphenyl)-4,5-diphenylimidazolium iodide based ionic liquid for biomass conversion into levulinic acid and formic acid.Bioresour Technol. 2020 Nov;315:123864. doi: 10.1016/j.biortech.2020.123864. Epub 2020 Jul 20. Bioresour Technol. 2020. PMID: 32711338
-
Catalytic Production of Levulinic Acid (LA) from Actual Biomass.Molecules. 2019 Jul 30;24(15):2760. doi: 10.3390/molecules24152760. Molecules. 2019. PMID: 31366018 Free PMC article. Review.
-
Homogeneous Catalyzed Reactions of Levulinic Acid: To γ-Valerolactone and Beyond.ChemSusChem. 2016 Aug 23;9(16):2037-47. doi: 10.1002/cssc.201600517. Epub 2016 Jul 28. ChemSusChem. 2016. PMID: 27464831 Review.
References
-
- Badgujar K.C., Badgujar V.C., Bhanage B.M. A review on catalytic synthesis of energy rich fuel additive levulinate compounds from biomass derived levulinic acid. Fuel Process. Technol. 2020;197:106213. doi: 10.1016/j.fuproc.2019.106213. - DOI
-
- Liu J., Li H., Liu Y.C., Lu Y.M., He J., Liu X.F., Wu Z.B., Yang S. Catalytic conversion of glucose to 5-hydroxymethylfurfural over nano-sized mesoporous Al2O3-B2O3 solid acids. Catal. Commun. 2015;62:19–23. doi: 10.1016/j.catcom.2015.01.008. - DOI
-
- Reddy P.S., Sudarsanam P., Raju G., Reddy B.M. Synthesis of bio-additives: Acetylation of glycerol over zirconia-based solid acid catalysts. Catal. Commun. 2010;11:1224–1228. doi: 10.1016/j.catcom.2010.07.006. - DOI
-
- Bhanja P., Modak A., Chatterjee S., Bhaumik A. Bifunctionalized Mesoporous SBA-15: A New Heterogeneous Catalyst for the Facile Synthesis of 5-Hydroxymethylfurfural. ACS Sustain. Chem. Eng. 2017;5:2763–2773. doi: 10.1021/acssuschemeng.6b03100. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

