Unraveling the In Vivo Protein Corona
- PMID: 33445454
- PMCID: PMC7826990
- DOI: 10.3390/cells10010132
Unraveling the In Vivo Protein Corona
Abstract
Understanding the behavior of nanoparticles upon contact with a physiological environment is of urgent need in order to improve their properties for a successful therapeutic application. Most commonly, the interaction of nanoparticles with plasma proteins are studied under in vitro conditions. However, this has been shown to not reflect the complex situation after in vivo administration. Therefore, here we focused on the investigation of magnetic nanoparticles with blood proteins under in vivo conditions. Importantly, we observed a radically different proteome in vivo in comparison to the in vitro situation underlining the significance of in vivo protein corona studies. Next to this, we found that the in vivo corona profile does not significantly change over time. To mimic the in vivo situation, we established an approach, which we termed "ex vivo" as it uses whole blood freshly prepared from an animal. Overall, we present a comprehensive analysis focusing on the interaction between nanoparticles and blood proteins under in vivo conditions and how to mimic this situation with our ex vivo approach. This knowledge is needed to characterize the true biological identity of nanoparticles.
Keywords: biodistribution; in vivo; nanoparticle; plasma; protein corona; serum.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



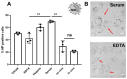
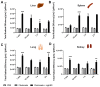
References
-
- Shen L., Tenzer S., Storck W., Hobernik D., Raker V.K., Fischer K., Decker S., Dzionek A., Krauthauser S., Diken M., et al. Protein corona-mediated targeting of nano-carriers to B cells allows redirection of allergic immune responses. J. Allergy Clin. Immunol. 2018;142:1558–1570. doi: 10.1016/j.jaci.2017.08.049. - DOI - PubMed
-
- Yallapu M.M., Chauhan N., Othman S.F., Khalilzad-Sharghi V., Ebeling M.C., Khan S., Jaggi M., Chauhan S.C. Implications of protein corona on physico-chemical and biological properties of magnetic nanoparticles. Biomaterials. 2015;46:1–12. doi: 10.1016/j.biomaterials.2014.12.045. - DOI - PMC - PubMed
-
- Monopoli M.P., Pitek A.S., Lynch I., Dawson K.A. Formation and characterization of the nanoparticle-protein corona. Methods Mol. Biol. 2013;1025:137–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

