Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense
- PMID: 33445463
- PMCID: PMC7826584
- DOI: 10.3390/microorganisms9010151
Cryptosporidial Infection Suppresses Intestinal Epithelial Cell MAPK Signaling Impairing Host Anti-Parasitic Defense
Abstract
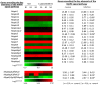
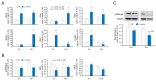
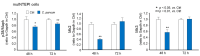
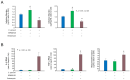
Cryptosporidium is a genus of protozoan parasites that infect the gastrointestinal epithelium of a variety of vertebrate hosts. Intestinal epithelial cells are the first line of defense and play a critical role in orchestrating host immunity against Cryptosporidium infection. To counteract host defense response, Cryptosporidium has developed strategies of immune evasion to promote parasitic replication and survival within epithelial cells, but the underlying mechanisms are largely unclear. Using various models of intestinal cryptosporidiosis, we found that Cryptosporidium infection caused suppression of mitogen-activated protein kinase (MAPK) signaling in infected murine intestinal epithelial cells. Whereas expression levels of most genes encoding the key components of the MAPK signaling pathway were not changed in infected intestinal epithelial cells, we detected a significant downregulation of p38/Mapk, MAP kinase-activated protein kinase 2 (Mk2), and Mk3 genes in infected host cells. Suppression of MAPK signaling was associated with an impaired intestinal epithelial defense against C. parvum infection. Our data suggest that cryptosporidial infection may suppress intestinal epithelial cell MAPK signaling associated with the evasion of host antimicrobial defense.
Keywords: Cryptosporidium; MAPK; cryptosporidiosis; defense; intestinal epithelium; p38/MAPK.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Checkley W., White A.C., Jr., Jaganath D., Arrowood M.J., Chalmers R.M., Chen X.M., Fayer R., Griffiths J.K., Guerrant R.L., Hedstrom L., et al. A review of the global burden, novel diagnostics, therapeutics, and vaccine targets for cryptosporidium. Lancet Infect. Dis. 2015;1:85–94. doi: 10.1016/S1473-3099(14)70772-8. - DOI - PMC - PubMed
-
- Kotloff K.L., Nataro J.P., Blackwelder W.C., Nasrin D., Farag T.H., Panchalingam S., Wu Y., Sow S.O., Sur D., Breiman R.F., et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the global enteric multicenter study, gems): A prospective, case-control study. Lancet. 2013;9888:209–222. doi: 10.1016/S0140-6736(13)60844-2. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

