Kynurenic Acid Electrochemical Immunosensor: Blood-Based Diagnosis of Alzheimer's Disease
- PMID: 33445512
- PMCID: PMC7827041
- DOI: 10.3390/bios11010020
Kynurenic Acid Electrochemical Immunosensor: Blood-Based Diagnosis of Alzheimer's Disease
Abstract
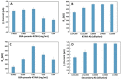
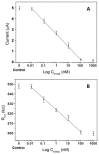
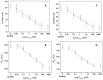
Alzheimer's disease (AD) is a neurodegenerative disorder, characterized by a functional deterioration of the brain. Currently, there are selected biomarkers for its diagnosis in cerebrospinal fluid. However, its extraction has several disadvantages for the patient. Therefore, there is an urgent need for a detection method using sensitive and selective blood-based biomarkers. Kynurenic acid (KYNA) is a potential biomarker candidate for this purpose. The alteration of the KYNA levels in blood has been related with inflammatory processes in the brain, produced as a protective function when neurons are damaged. This paper describes a novel electrochemical immunosensor for KYNA detection, based on successive functionalization multi-electrode array. The resultant sensor was characterized by cyclic voltammetry (CV), chronoamperometry (CA), and electrochemical impedance spectroscopy (EIS). The proposed biosensor detects KYNA within a linear calibration range from 10 pM to 100 nM using CA and EIS, obtaining a limit of detection (LOD) of 16.9 pM and 37.6 pM in buffer, respectively, being the lowest reported LOD for this biomarker. Moreover, to assess our device closer to the real application, the developed immunosensor was also tested under human serum matrix, obtaining an LOD of 391.71 pM for CA and 278.8 pM for EIS with diluted serum.
Keywords: Alzheimer’s disease (AD); blood analysis; chronoamperometry (CA); electrochemical biosensor; electrochemical impedance spectroscopy (EIS); immunosensor; in vitro diagnosis (IVD); kynurenic acid (KYNA); point of care diagnosis (PoC).
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Earlier Detection of Alzheimer's Disease Based on a Novel Biomarker cis P-tau by a Label-Free Electrochemical Immunosensor.Biosensors (Basel). 2022 Oct 17;12(10):879. doi: 10.3390/bios12100879. Biosensors (Basel). 2022. PMID: 36291017 Free PMC article.
-
Competitive electrochemical immunosensor for the detection of unfolded p53 protein in blood as biomarker for Alzheimer's disease.Anal Chim Acta. 2020 Jan 6;1093:28-34. doi: 10.1016/j.aca.2019.09.042. Epub 2019 Sep 16. Anal Chim Acta. 2020. PMID: 31735212
-
A novel silanization agent based single used biosensing system: Detection of C-reactive protein as a potential Alzheimer's disease blood biomarker.J Pharm Biomed Anal. 2018 May 30;154:227-235. doi: 10.1016/j.jpba.2018.03.016. Epub 2018 Mar 12. J Pharm Biomed Anal. 2018. PMID: 29558723
-
Nano-biosensors to detect beta-amyloid for Alzheimer's disease management.Biosens Bioelectron. 2016 Jun 15;80:273-287. doi: 10.1016/j.bios.2016.01.065. Epub 2016 Jan 28. Biosens Bioelectron. 2016. PMID: 26851586 Free PMC article. Review.
-
Electrochemical biosensors for early detection of Alzheimer's disease.Clin Chim Acta. 2025 May 15;572:120278. doi: 10.1016/j.cca.2025.120278. Epub 2025 Apr 2. Clin Chim Acta. 2025. PMID: 40185381 Review.
Cited by
-
Unlocking the Secrets: Exploring the Biochemical Correlates of Suicidal Thoughts and Behaviors in Adults with Autism Spectrum Conditions.Biomedicines. 2023 May 31;11(6):1600. doi: 10.3390/biomedicines11061600. Biomedicines. 2023. PMID: 37371695 Free PMC article.
-
Transformative biomedical devices to overcome biomatrix effects.Biosens Bioelectron. 2025 Jul 1;279:117373. doi: 10.1016/j.bios.2025.117373. Epub 2025 Mar 12. Biosens Bioelectron. 2025. PMID: 40120290 Review.
-
Sensitive Detection of Kynurenic Acid from Biological Fluids Using a Flexible Electrochemical Platform Based on Gold Nanoparticles and Reduced Graphene Oxide.Int J Mol Sci. 2025 Jan 22;26(3):913. doi: 10.3390/ijms26030913. Int J Mol Sci. 2025. PMID: 39940684 Free PMC article.
-
A reagent-free, fouling-resistant electrochemical multifunctional peptide sensor for highly sensitive and rapid detection of Alzheimer's disease biomarkers.Mater Today Bio. 2025 Jul 12;33:102081. doi: 10.1016/j.mtbio.2025.102081. eCollection 2025 Aug. Mater Today Bio. 2025. PMID: 40697322 Free PMC article.
-
Electrochemical Determination of Kynurenine Pathway Metabolites-Challenges and Perspectives.Sensors (Basel). 2021 Oct 28;21(21):7152. doi: 10.3390/s21217152. Sensors (Basel). 2021. PMID: 34770460 Free PMC article. Review.
References
-
- Alzheimer’s Disease International (ADI), London, The State of the Art of Dementia Research: New Frontiers. September 2018. [(accessed on 3 January 2021)]; Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2018.pdf.
-
- World Health Organiztion Dementia Key Facts. 21 September 2020. [(accessed on 3 January 2021)]; Available online: https://www.who.int/news-room/fact-sheets/detail/dementia#:~:text=Worldw....
-
- Šimić G., Babić Leko M., Wray S., Harrington C., Delalle I., Jovanov-Milošević N., Bažadona D., Buée L., de Silva R., Giovanni G., et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules. 2016;6:1–28. - PMC - PubMed
-
- Gordon B.A., Blazey T.M., Su Y., Hari-Raj A., Dincer A., Flores S., Christensen J., McDade E., Wang G., Xiong C., et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018;17:241–250. doi: 10.1016/S1474-4422(18)30028-0. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

