In Situ "Humanization" of Porcine Bioprostheses: Demonstration of Tendon Bioprostheses Conversion into Human ACL and Possible Implications for Heart Valve Bioprostheses
- PMID: 33445522
- PMCID: PMC7826727
- DOI: 10.3390/bioengineering8010010
In Situ "Humanization" of Porcine Bioprostheses: Demonstration of Tendon Bioprostheses Conversion into Human ACL and Possible Implications for Heart Valve Bioprostheses
Abstract
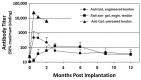
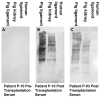
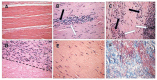
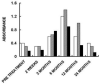
This review describes the first studies on successful conversion of porcine soft-tissue bioprostheses into viable permanently functional tissue in humans. This process includes gradual degradation of the porcine tissue, with concomitant neo-vascularization and reconstruction of the implanted bioprosthesis with human cells and extracellular matrix. Such a reconstruction process is referred to in this review as "humanization". Humanization was achieved with porcine bone-patellar-tendon-bone (BTB), replacing torn anterior-cruciate-ligament (ACL) in patients. In addition to its possible use in orthopedic surgery, it is suggested that this humanization method should be studied as a possible mechanism for converting implanted porcine bioprosthetic heart-valves (BHV) into viable tissue valves in young patients. Presently, these patients are only implanted with mechanical heart-valves, which require constant anticoagulation therapy. The processing of porcine bioprostheses, which enables humanization, includes elimination of α-gal epitopes and partial (incomplete) crosslinking with glutaraldehyde. Studies on implantation of porcine BTB bioprostheses indicated that enzymatic elimination of α-gal epitopes prevents subsequent accelerated destruction of implanted tissues by the natural anti-Gal antibody, whereas the partial crosslinking by glutaraldehyde molecules results in their function as "speed bumps" that slow the infiltration of macrophages. Anti-non gal antibodies produced against porcine antigens in implanted bioprostheses recruit macrophages, which infiltrate at a pace that enables slow degradation of the porcine tissue, neo-vascularization, and infiltration of fibroblasts. These fibroblasts align with the porcine collagen-fibers scaffold, secrete their collagen-fibers and other extracellular-matrix (ECM) components, and gradually replace porcine tissues degraded by macrophages with autologous functional viable tissue. Porcine BTB implanted in patients completes humanization into autologous ACL within ~2 years. The similarities in cells and ECM comprising heart-valves and tendons, raises the possibility that porcine BHV undergoing a similar processing, may also undergo humanization, resulting in formation of an autologous, viable, permanently functional, non-calcifying heart-valves.
Keywords: anterior cruciate ligament reconstruction; anti-Gal antibody; anti-non gal antibody; bioprosthesis humanization; heart valve bioprosthesis; porcine tendon bioprosthesis; α-gal epitope; α-galactosidase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Induced Remodeling of Porcine Tendons to Human Anterior Cruciate Ligaments by α-GAL Epitope Removal and Partial Cross-Linking.Tissue Eng Part B Rev. 2017 Aug;23(4):412-419. doi: 10.1089/ten.TEB.2016.0332. Epub 2017 Feb 14. Tissue Eng Part B Rev. 2017. PMID: 28068870 Free PMC article.
-
Alpha-Gal Inactivated Heart Valve Bioprostheses Exhibit an Anti-Calcification Propensity Similar to Knockout Tissues<sup/>Tissue Eng Part A. 2017 Oct;23(19-20):1181-1195. doi: 10.1089/ten.tea.2016.0474. Epub 2017 Jun 21. Tissue Eng Part A. 2017. PMID: 29053434
-
The Immune Responses and Calcification of Bioprostheses in the α1,3-Galactosyltransferase Knockout Mouse.J Heart Valve Dis. 2016 Mar;25(2):253-261. J Heart Valve Dis. 2016. PMID: 27989076
-
Founder's Award, 25th Annual Meeting of the Society for Biomaterials, perspectives. Providence, RI, April 28-May 2, 1999. Tissue heart valves: current challenges and future research perspectives.J Biomed Mater Res. 1999 Dec 15;47(4):439-65. doi: 10.1002/(sici)1097-4636(19991215)47:4<439::aid-jbm1>3.0.co;2-o. J Biomed Mater Res. 1999. PMID: 10497280 Review.
-
Avoiding detrimental human immune response against Mammalian extracellular matrix implants.Tissue Eng Part B Rev. 2015 Apr;21(2):231-41. doi: 10.1089/ten.TEB.2014.0392. Epub 2014 Nov 20. Tissue Eng Part B Rev. 2015. PMID: 25315097 Review.
Cited by
-
Immunogenicity of decellularized extracellular matrix scaffolds: a bottleneck in tissue engineering and regenerative medicine.Biomater Res. 2023 Feb 9;27(1):10. doi: 10.1186/s40824-023-00348-z. Biomater Res. 2023. PMID: 36759929 Free PMC article. Review.
-
Can Heart Valve Decellularization Be Standardized? A Review of the Parameters Used for the Quality Control of Decellularization Processes.Front Bioeng Biotechnol. 2022 Feb 17;10:830899. doi: 10.3389/fbioe.2022.830899. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35252139 Free PMC article. Review.
-
Advances in Regenerative Sports Medicine Research.Front Bioeng Biotechnol. 2022 May 13;10:908751. doi: 10.3389/fbioe.2022.908751. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 35646865 Free PMC article. Review.
-
Xenograft bone-patellar tendon-bone ACL reconstruction: a case series at 20-year follow-up as proof of principle.J Exp Orthop. 2023 Sep 6;10(1):91. doi: 10.1186/s40634-023-00651-7. J Exp Orthop. 2023. PMID: 37672199 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

