The Vascular Effects of Isolated Isoflavones-A Focus on the Determinants of Blood Pressure Regulation
- PMID: 33445531
- PMCID: PMC7827317
- DOI: 10.3390/biology10010049
The Vascular Effects of Isolated Isoflavones-A Focus on the Determinants of Blood Pressure Regulation
Abstract
Isoflavones are phytoestrogen compounds with important biological activities, including improvement of cardiovascular health. This activity is most evident in populations with a high isoflavone dietary intake, essentially from soybean-based products. The major isoflavones known to display the most important cardiovascular effects are genistein, daidzein, glycitein, formononetin, and biochanin A, although the closely related metabolite equol is also relevant. Most clinical studies have been focused on the impact of dietary intake or supplementation with mixtures of compounds, with only a few addressing the effect of isolated compounds. This paper reviews the main actions of isolated isoflavones on the vasculature, with particular focus given to their effect on the determinants of blood pressure regulation. Isoflavones exert vasorelaxation due to a multitude of pathways in different vascular beds. They can act in the endothelium to potentiate the release of NO and endothelium-derived hyperpolarization factors. In the vascular smooth muscle, isoflavones modulate calcium and potassium channels, leading to hyperpolarization and relaxation. Some of these effects are influenced by the binding of isoflavones to estrogen receptors and to the inhibition of specific kinase enzymes. The vasorelaxation effects of isoflavones are mostly obtained with plasma concentrations in the micromolar range, which are only attained through supplementation. This paper highlights isolated isoflavones as potentially suitable alternatives to soy-based foodstuffs and supplements and which could enlarge the current therapeutic arsenal. Nonetheless, more studies are needed to better establish their safety profile and elect the most useful applications.
Keywords: endothelium; estrogen receptor; ion channels; isoflavones; tyrosine kinase; vasorelaxation.
Conflict of interest statement
The author declares no conflict of interest.
Figures
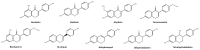
Similar articles
-
Bioavailability of pure isoflavones in healthy humans and analysis of commercial soy isoflavone supplements.J Nutr. 2001 Apr;131(4 Suppl):1362S-75S. doi: 10.1093/jn/131.4.1362S. J Nutr. 2001. PMID: 11285356
-
Formononetin, an isoflavone, relaxes rat isolated aorta through endothelium-dependent and endothelium-independent pathways.J Nutr Biochem. 2010 Jul;21(7):613-20. doi: 10.1016/j.jnutbio.2009.03.010. Epub 2009 Jun 30. J Nutr Biochem. 2010. PMID: 19570671
-
Impact of equol-producing capacity and soy-isoflavone profiles of supplements on bone calcium retention in postmenopausal women: a randomized crossover trial.Am J Clin Nutr. 2015 Sep;102(3):695-703. doi: 10.3945/ajcn.114.093906. Epub 2015 Aug 5. Am J Clin Nutr. 2015. PMID: 26245807 Free PMC article. Clinical Trial.
-
Isoflavones.Molecules. 2019 Mar 19;24(6):1076. doi: 10.3390/molecules24061076. Molecules. 2019. PMID: 30893792 Free PMC article. Review.
-
Potential Protective Mechanisms of S-equol, a Metabolite of Soy Isoflavone by the Gut Microbiome, on Cognitive Decline and Dementia.Int J Mol Sci. 2022 Oct 7;23(19):11921. doi: 10.3390/ijms231911921. Int J Mol Sci. 2022. PMID: 36233223 Free PMC article. Review.
Cited by
-
Exploring the Complex Mechanisms of Isoflavones: From Cell Bioavailability, to Cell Dynamics and Breast Cancer.Phytother Res. 2025 Feb;39(2):957-979. doi: 10.1002/ptr.8417. Epub 2024 Dec 20. Phytother Res. 2025. PMID: 39707600 Free PMC article. Review.
-
Inhibition of α1-Adrenergic, Non-Adrenergic and Neurogenic Human Prostate Smooth Muscle Contraction and of Stromal Cell Growth by the Isoflavones Genistein and Daidzein.Nutrients. 2022 Nov 22;14(23):4943. doi: 10.3390/nu14234943. Nutrients. 2022. PMID: 36500973 Free PMC article.
-
Three Classes of Antioxidant Defense Systems and the Development of Postmenopausal Osteoporosis.Front Physiol. 2022 Mar 3;13:840293. doi: 10.3389/fphys.2022.840293. eCollection 2022. Front Physiol. 2022. PMID: 35309045 Free PMC article. Review.
-
Chlamydomonas agloeformis from the Ecuadorian Highlands: Nutrients and Bioactive Compounds Profiling and In Vitro Antioxidant Activity.Foods. 2023 Aug 22;12(17):3147. doi: 10.3390/foods12173147. Foods. 2023. PMID: 37685081 Free PMC article.
-
Arrest of mouse preterm labor until term delivery by combination therapy with atosiban and mundulone, a natural product with tocolytic efficacy.Pharmacol Res. 2023 Sep;195:106876. doi: 10.1016/j.phrs.2023.106876. Epub 2023 Aug 1. Pharmacol Res. 2023. PMID: 37536638 Free PMC article.
References
-
- Xu X., Duncan A.M., Merz B.E., Kurzer M.S. Effects of soy isoflavones on estrogen and phytoestrogen metabolism in premenopausal women. Cancer Epidemiol. Biomarkers Prev. 1998;7:1101–1108. - PubMed
-
- Dakora F.D., Phillips D.A. Diverse functions of isoflavonoids in legumes transcend anti-microbial definitions of phytoalexins. Physiol. Mol. Plant Pathol. 1996;49:1–20. doi: 10.1006/pmpp.1996.0035. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

