Synthesis and Biochemical Evaluation of Warhead-Decorated Psoralens as (Immuno)Proteasome Inhibitors
- PMID: 33445542
- PMCID: PMC7826781
- DOI: 10.3390/molecules26020356
Synthesis and Biochemical Evaluation of Warhead-Decorated Psoralens as (Immuno)Proteasome Inhibitors
Abstract
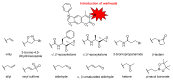
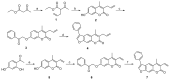
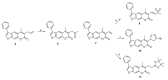
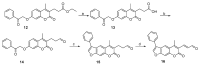
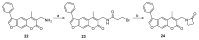
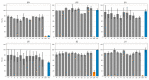
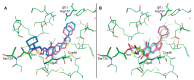
The immunoproteasome is a multicatalytic protease that is predominantly expressed in cells of hematopoietic origin. Its elevated expression has been associated with autoimmune diseases, various types of cancer, and inflammatory diseases. Selective inhibition of its catalytic activities is therefore a viable approach for the treatment of these diseases. However, the development of immunoproteasome-selective inhibitors with non-peptidic scaffolds remains a challenging task. We previously reported 7H-furo[3,2-g]chromen-7-one (psoralen)-based compounds with an oxathiazolone warhead as selective inhibitors of the chymotrypsin-like (β5i) subunit of immunoproteasome. Here, we describe the influence of the electrophilic warhead variations at position 3 of the psoralen core on the inhibitory potencies. Despite mapping the chemical space with different warheads, all compounds showed decreased inhibition of the β5i subunit of immunoproteasome in comparison to the parent oxathiazolone-based compound. Although suboptimal, these results provide crucial information about structure-activity relationships that will serve as guidance for the further design of (immuno)proteasome inhibitors.
Keywords: electrophilic compounds; immunoproteasome; non-peptidic; psoralen core; warhead scan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
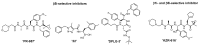
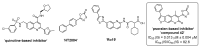
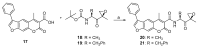
Similar articles
-
Discovery of selective fragment-sized immunoproteasome inhibitors.Eur J Med Chem. 2021 Jul 5;219:113455. doi: 10.1016/j.ejmech.2021.113455. Epub 2021 Apr 20. Eur J Med Chem. 2021. PMID: 33894528
-
A focused structure-activity relationship study of psoralen-based immunoproteasome inhibitors.Medchemcomm. 2019 Sep 13;10(11):1958-1965. doi: 10.1039/c9md00365g. eCollection 2019 Nov 1. Medchemcomm. 2019. PMID: 32952997 Free PMC article.
-
Fragment-Sized and Bidentate (Immuno)Proteasome Inhibitors Derived from Cysteine and Threonine Targeting Warheads.Cells. 2021 Dec 6;10(12):3431. doi: 10.3390/cells10123431. Cells. 2021. PMID: 34943940 Free PMC article.
-
A patent review of immunoproteasome inhibitors.Expert Opin Ther Pat. 2018 Jul;28(7):517-540. doi: 10.1080/13543776.2018.1484904. Epub 2018 Jun 14. Expert Opin Ther Pat. 2018. PMID: 29865878 Review.
-
Immunoproteasome-selective inhibitors: An overview of recent developments as potential drugs for hematologic malignancies and autoimmune diseases.Eur J Med Chem. 2019 Nov 15;182:111646. doi: 10.1016/j.ejmech.2019.111646. Epub 2019 Aug 29. Eur J Med Chem. 2019. PMID: 31521028 Review.
Cited by
-
Coumarin and Its Derivatives-Editorial.Molecules. 2021 Oct 19;26(20):6320. doi: 10.3390/molecules26206320. Molecules. 2021. PMID: 34684900 Free PMC article.
-
Syntheses, reactivity, and biological applications of coumarins.Front Chem. 2024 Feb 19;12:1362992. doi: 10.3389/fchem.2024.1362992. eCollection 2024. Front Chem. 2024. PMID: 38440776 Free PMC article. Review.
-
Recent Advances in the Development of Immunoproteasome Inhibitors as Anti-Cancer Agents: The Past 5 Years.Molecules. 2025 Feb 6;30(3):755. doi: 10.3390/molecules30030755. Molecules. 2025. PMID: 39942858 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

