Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson's Disease
- PMID: 33445611
- PMCID: PMC7827283
- DOI: 10.3390/molecules26020361
Cytokinin Plant Hormones Have Neuroprotective Activity in In Vitro Models of Parkinson's Disease
Abstract
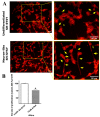
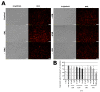
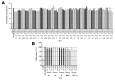
Cytokinins are adenine-based phytohormones that regulate key processes in plants, such as cell division and differentiation, root and shoot growth, apical dominance, branching, and seed germination. In preliminary studies, they have also shown protective activities against human neurodegenerative diseases. To extend knowledge of the protection (protective activity) they offer, we investigated activities of natural cytokinins against salsolinol (SAL)-induced toxicity (a Parkinson's disease model) and glutamate (Glu)-induced death of neuron-like dopaminergic SH-SY5Y cells. We found that kinetin-3-glucoside, cis-zeatin riboside, and N6-isopentenyladenosine were active in the SAL-induced PD model. In addition, trans-, cis-zeatin, and kinetin along with the iron chelator deferoxamine (DFO) and the necroptosis inhibitor necrostatin 1 (NEC-1) significantly reduced cell death rates in the Glu-induced model. Lactate dehydrogenase assays revealed that the cytokinins provided lower neuroprotective activity than DFO and NEC-1. Moreover, they reduced apoptotic caspase-3/7 activities less strongly than DFO. However, the cytokinins had very similar effects to DFO and NEC-1 on superoxide radical production. Overall, they showed protective activity in the SAL-induced model of parkinsonian neuronal cell death and Glu-induced model of oxidative damage mainly by reduction of oxidative stress.
Keywords: Parkinson’s disease; cytokinin; cytotoxicity; glutamate; neuron-like SH-SY5Y cells; neuroprotection; oxidative stress; phytohormone; salsolinol.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Neuroprotective Effects of Necrostatin-1 Against Oxidative Stress-Induced Cell Damage: an Involvement of Cathepsin D Inhibition.Neurotox Res. 2020 Mar;37(3):525-542. doi: 10.1007/s12640-020-00164-6. Epub 2020 Jan 21. Neurotox Res. 2020. PMID: 31960265 Free PMC article.
-
Targeting Ferroptosis: Acteoside as a Neuroprotective Agent in Salsolinol-Induced Parkinson's Disease Models.Front Biosci (Landmark Ed). 2025 Feb 14;30(2):26679. doi: 10.31083/FBL26679. Front Biosci (Landmark Ed). 2025. PMID: 40018928
-
Endogenous cytokinin profiles of tissue-cultured and acclimatized 'Williams' bananas subjected to different aromatic cytokinin treatments.Plant Sci. 2014 Jan;214:88-98. doi: 10.1016/j.plantsci.2013.09.012. Epub 2013 Oct 2. Plant Sci. 2014. PMID: 24268166
-
Neuroprotection by propargylamines in Parkinson's disease: suppression of apoptosis and induction of prosurvival genes.Neurotoxicol Teratol. 2002 Sep-Oct;24(5):675-82. doi: 10.1016/s0892-0362(02)00221-0. Neurotoxicol Teratol. 2002. PMID: 12200198 Review.
-
Differentiation of human myeloid leukemia cells by plant redifferentiation-inducing hormones.Leuk Lymphoma. 2002 Sep;43(9):1729-35. doi: 10.1080/1042819021000006493. Leuk Lymphoma. 2002. PMID: 12685824 Review.
Cited by
-
Allies or Enemies? The Power of Plant Hormones in Animals: Insights into Their Regulatory Roles.Molecules. 2025 Jul 16;30(14):2984. doi: 10.3390/molecules30142984. Molecules. 2025. PMID: 40733250 Free PMC article. Review.
-
Fungal-Mediated Biotransformation of the Plant Growth Regulator Forchlorfenuron by Cunninghamella elegans.Metabolites. 2024 Feb 1;14(2):101. doi: 10.3390/metabo14020101. Metabolites. 2024. PMID: 38392993 Free PMC article.
-
Photoprotective properties of new derivatives of kinetin.Photochem Photobiol Sci. 2023 Feb;22(2):357-369. doi: 10.1007/s43630-022-00320-1. Epub 2022 Oct 20. Photochem Photobiol Sci. 2023. PMID: 36264480
-
Unscrambling the Role of Redox-Active Biometals in Dopaminergic Neuronal Death and Promising Metal Chelation-Based Therapy for Parkinson's Disease.Int J Mol Sci. 2023 Jan 9;24(2):1256. doi: 10.3390/ijms24021256. Int J Mol Sci. 2023. PMID: 36674772 Free PMC article. Review.
-
Cytokinins: Wide-Spread Signaling Hormones from Plants to Humans with High Medical Potential.Nutrients. 2022 Apr 2;14(7):1495. doi: 10.3390/nu14071495. Nutrients. 2022. PMID: 35406107 Free PMC article. Review.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

