Molecular Properties of Bare and Microhydrated Vitamin B5-Calcium Complexes
- PMID: 33445631
- PMCID: PMC7826572
- DOI: 10.3390/ijms22020692
Molecular Properties of Bare and Microhydrated Vitamin B5-Calcium Complexes
Abstract
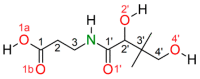
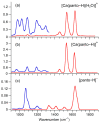
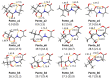
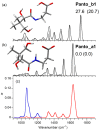
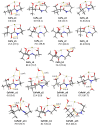
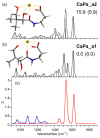
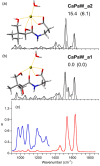
Pantothenic acid, also called vitamin B5, is an essential nutrient involved in several metabolic pathways. It shows a characteristic preference for interacting with Ca(II) ions, which are abundant in the extracellular media and act as secondary mediators in the activation of numerous biological functions. The bare deprotonated form of pantothenic acid, [panto-H]-, its complex with Ca(II) ion, [Ca(panto-H)]+, and singly charged micro-hydrated calcium pantothenate [Ca(panto-H)(H2O)]+ adduct have been obtained in the gas phase by electrospray ionization and assayed by mass spectrometry and IR multiple photon dissociation spectroscopy in the fingerprint spectral range. Quantum chemical calculations at the B3LYP(-D3) and MP2 levels of theory were performed to simulate geometries, thermochemical data, and linear absorption spectra of low-lying isomers, allowing us to assign the experimental absorptions to particular structural motifs. Pantothenate was found to exist in the gas phase as a single isomeric form showing deprotonation on the carboxylic moiety. On the contrary, free and monohydrated calcium complexes of deprotonated pantothenic acid both present at least two isomers participating in the gas-phase population, sharing the deprotonation of pantothenate on the carboxylic group and either a fourfold or fivefold coordination with calcium, thus justifying the strong affinity of pantothenate for the metal.
Keywords: IRMPD spectroscopy; calcium; complexation; molecular structure; pantothenate; quantum chemistry calculations; vitamin B5.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
Similar articles
-
Gas phase structure and reactivity of doubly charged microhydrated calcium(II)-catechol complexes probed by infrared spectroscopy.J Phys Chem A. 2014 Jul 10;118(27):4942-54. doi: 10.1021/jp503789j. Epub 2014 Jun 25. J Phys Chem A. 2014. PMID: 24963704
-
Structures of alkali metal ion-adenine complexes and hydrated complexes by IRMPD spectroscopy and electronic structure calculations.J Phys Chem A. 2010 Mar 18;114(10):3449-56. doi: 10.1021/jp9098683. J Phys Chem A. 2010. PMID: 20163169
-
Structures of bare and hydrated [Pb(aminoacid-H)]+ complexes using infrared multiple photon dissociation spectroscopy.J Phys Chem B. 2011 Oct 6;115(39):11506-18. doi: 10.1021/jp2068655. Epub 2011 Sep 15. J Phys Chem B. 2011. PMID: 21875029
-
Vibrational spectroscopy of bare and solvated ionic complexes of biological relevance.Mass Spectrom Rev. 2009 May-Jun;28(3):468-94. doi: 10.1002/mas.20215. Mass Spectrom Rev. 2009. PMID: 19241457 Review.
-
Application of Infrared Multiple Photon Dissociation (IRMPD) Spectroscopy in Chiral Analysis.Molecules. 2020 Nov 5;25(21):5152. doi: 10.3390/molecules25215152. Molecules. 2020. PMID: 33167464 Free PMC article. Review.
Cited by
-
Sotorasib-impaired degradation of NEU1 contributes to cardiac injury by inhibiting AKT signaling.Cell Death Discov. 2025 Apr 12;11(1):169. doi: 10.1038/s41420-025-02431-x. Cell Death Discov. 2025. PMID: 40221400 Free PMC article.
-
Anharmonicity and Spectra-Structure Correlations in MIR and NIR Spectra of Crystalline Menadione (Vitamin K3).Molecules. 2021 Nov 10;26(22):6779. doi: 10.3390/molecules26226779. Molecules. 2021. PMID: 34833871 Free PMC article.
-
Metabolomics analysis of the effects of chelerythrine on Ustilaginoidea virens.J Pestic Sci. 2024 May 20;49(2):104-113. doi: 10.1584/jpestics.D23-065. J Pestic Sci. 2024. PMID: 38882710 Free PMC article.
-
Impact of Sulfur Fumigation on the Chemistry of Dioscoreae Rhizoma (Chinese Yam).ACS Omega. 2023 May 30;8(23):21293-21304. doi: 10.1021/acsomega.3c02729. eCollection 2023 Jun 13. ACS Omega. 2023. PMID: 37332814 Free PMC article.
-
Molecular Basis for the Remarkably Different Gas-Phase Behavior of Deprotonated Thyroid Hormones Triiodothyronine (T3) and Reverse Triiodothyronine (rT3): A Clue for Their Discrimination?Anal Chem. 2021 Nov 9;93(44):14869-14877. doi: 10.1021/acs.analchem.1c03892. Epub 2021 Oct 29. Anal Chem. 2021. PMID: 34714056 Free PMC article.
References
-
- Kohlmeier M. Nutrigenetics. Elsevier; Amsterdam, The Netherlands: 2013. How Nutrients are Affected by Genetics; pp. 103–221.
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

