Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs
- PMID: 33445698
- PMCID: PMC7828127
- DOI: 10.3390/nu13010205
Parenteral Fish-Oil Containing Lipid Emulsions Limit Initial Lipopolysaccharide-Induced Host Immune Responses in Preterm Pigs
Abstract
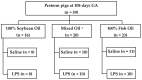
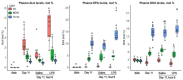
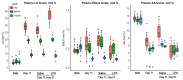
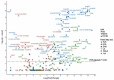
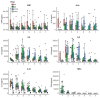
Multicomponent lipid emulsions are available for critical care of preterm infants. We sought to determine the impact of different lipid emulsions on early priming of the host and its response to an acute stimulus. Pigs delivered 7d preterm (n = 59) were randomized to receive different lipid emulsions for 11 days: 100% soybean oil (SO), mixed oil emulsion (SO, medium chain olive oil and fish oil) including 15% fish oil (MO15), or 100% fish oil (FO100). On day 11, pigs received an 8-h continuous intravenous infusion of either lipopolysaccharide (LPS-lyophilized Escherichia coli) or saline. Plasma was collected for fatty acid, oxylipin, metabolomic, and cytokine analyses. At day 11, plasma omega-3 fatty acid levels in the FO100 groups showed the highest increase in eicosapentaenoic acid, EPA (0.1 ± 0.0 to 9.7 ± 1.9, p < 0.001), docosahexaenoic acid, DHA (day 0 = 2.5 ± 0.7 to 13.6 ± 2.9, p < 0.001), EPA and DHA-derived oxylipins, and sphingomyelin metabolites. In the SO group, levels of cytokine IL1β increased at the first hour of LPS infusion (296.6 ± 308 pg/mL) but was undetectable in MO15, FO100, or in the animals receiving saline instead of LPS. Pigs in the SO group showed a significant increase in arachidonic acid (AA)-derived prostaglandins and thromboxanes in the first hour (p < 0.05). No significant changes in oxylipins were observed with either fish-oil containing group during LPS infusion. Host priming with soybean oil in the early postnatal period preserves a higher AA:DHA ratio and the ability to acutely respond to an external stimulus. In contrast, fish-oil containing lipid emulsions increase DHA, exacerbate a deficit in AA, and limit the initial LPS-induced inflammatory responses in preterm pigs.
Keywords: acute inflammation; arachidonic acid; eicosanoids; fatty acids; lipid metabolism; metabolomics; oxylipins; preterm pig; sphingomyelin.
Conflict of interest statement
Fresenius Kabi provided the lipid emulsions used in this study. Martin has grant support from Feihe International and Mead Johnson Nutrition; serves on the scientific advisory boards of Plakous Therapeutics, Inc. and LUCA Biologics; and has served as a consultant to Fresenius Kabi. Burrin has received grant support from Fresenius Kabi outside of this work. The other authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures




References
-
- Martin C.R., Dasilva D.A., Cluette B.J.E., Dimonda C., Hamill A., Bhutta A.Q., Coronel E., Wilschanski M., Stephens A.J., Driscoll D.F., et al. Decreased postnatal docosahexaenoic and arachidonic acid blood levels in premature infants are associated with neonatal morbidities. J. Pediatr. 2011;159:743–749. doi: 10.1016/j.jpeds.2011.04.039. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

