In Vivo Metabolism of [1,6-13C2]Glucose Reveals Distinct Neuroenergetic Functionality between Mouse Hippocampus and Hypothalamus
- PMID: 33445747
- PMCID: PMC7828183
- DOI: 10.3390/metabo11010050
In Vivo Metabolism of [1,6-13C2]Glucose Reveals Distinct Neuroenergetic Functionality between Mouse Hippocampus and Hypothalamus
Abstract
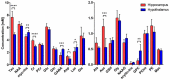
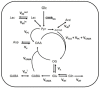
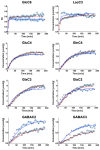
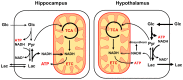
Glucose is a major energy fuel for the brain, however, less is known about specificities of its metabolism in distinct cerebral areas. Here we examined the regional differences in glucose utilization between the hypothalamus and hippocampus using in vivo indirect 13C magnetic resonance spectroscopy (1H-[13C]-MRS) upon infusion of [1,6-13C2]glucose. Using a metabolic flux analysis with a 1-compartment mathematical model of brain metabolism, we report that compared to hippocampus, hypothalamus shows higher levels of aerobic glycolysis associated with a marked gamma-aminobutyric acid-ergic (GABAergic) and astrocytic metabolic dependence. In addition, our analysis suggests a higher rate of ATP production in hypothalamus that is accompanied by an excess of cytosolic nicotinamide adenine dinucleotide (NADH) production that does not fuel mitochondria via the malate-aspartate shuttle (MAS). In conclusion, our results reveal significant metabolic differences, which might be attributable to respective cell populations or functional features of both structures.
Keywords: 13C-MRS; brain metabolism; in vivo imaging; mathematical modeling; metabolic flux analysis.
Conflict of interest statement
The authors declare no conflict of interest. Wuhan United Imaging Life Science Instruments Ltd. has played no role in this research.
Figures

Similar articles
-
Probing the cardiac malate-aspartate shuttle non-invasively using hyperpolarized [1,2-13 C2 ]pyruvate.NMR Biomed. 2018 Jan;31(1). doi: 10.1002/nbm.3845. Epub 2017 Nov 6. NMR Biomed. 2018. PMID: 29106770
-
In vivo 13C MRS in the mouse brain at 14.1 Tesla and metabolic flux quantification under infusion of [1,6-13C2]glucose.J Cereb Blood Flow Metab. 2018 Oct;38(10):1701-1714. doi: 10.1177/0271678X17734101. Epub 2017 Oct 19. J Cereb Blood Flow Metab. 2018. PMID: 29047296 Free PMC article.
-
In vivo characterization of brain metabolism by 1 H MRS, 13 C MRS and 18 FDG PET reveals significant glucose oxidation of invasively growing glioma cells.Int J Cancer. 2018 Jul 1;143(1):127-138. doi: 10.1002/ijc.31299. Epub 2018 Feb 21. Int J Cancer. 2018. PMID: 29417580
-
How Energy Metabolism Supports Cerebral Function: Insights from 13C Magnetic Resonance Studies In vivo.Front Neurosci. 2017 May 26;11:288. doi: 10.3389/fnins.2017.00288. eCollection 2017. Front Neurosci. 2017. PMID: 28603480 Free PMC article. Review.
-
Aerobic glycolysis during brain activation: adrenergic regulation and influence of norepinephrine on astrocytic metabolism.J Neurochem. 2016 Jul;138(1):14-52. doi: 10.1111/jnc.13630. Epub 2016 Jun 10. J Neurochem. 2016. PMID: 27166428 Review.
Cited by
-
INCA 2.0: A tool for integrated, dynamic modeling of NMR- and MS-based isotopomer measurements and rigorous metabolic flux analysis.Metab Eng. 2022 Jan;69:275-285. doi: 10.1016/j.ymben.2021.12.009. Epub 2021 Dec 26. Metab Eng. 2022. PMID: 34965470 Free PMC article.
References
-
- Kleinridders A., Ferris H.A., Reyzer M.L., Rath M., Soto M., Manier M.L., Spraggins J.M., Yang Z., Stanton R.C., Caprioli R.M., et al. Regional differences in brain glucose metabolism determined by imaging mass spectrometry. Mol. Metab. 2018;12:113–121. doi: 10.1016/j.molmet.2018.03.013. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

