Activation of an Effective Immune Response after Yellow Fever Vaccination Is Associated with the Genetic Background and Early Response of IFN-γ and CLEC5A
- PMID: 33445752
- PMCID: PMC7828179
- DOI: 10.3390/v13010096
Activation of an Effective Immune Response after Yellow Fever Vaccination Is Associated with the Genetic Background and Early Response of IFN-γ and CLEC5A
Abstract
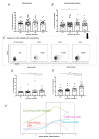
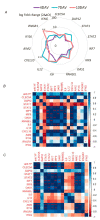
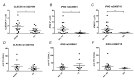
The yellow fever vaccine (YF17DD) is highly effective with a single injection conferring protection for at least 10 years. The YF17DD induces polyvalent responses, with a TH1/TH2 CD4+ profile, robust T CD8+ responses, and synthesis of interferon-gamma (IFN-γ), culminating in high titers of neutralizing antibodies. Furthermore, C-type lectin domain containing 5A (CLEC5A) has been implicated in innate outcomes in other flaviviral infections. Here, we conducted a follow-up study in volunteers immunized with YF17DD, investigating the humoral response, cellular phenotypes, gene expression, and single nucleotide polymorphisms (SNPs) of IFNG and CLEC5A, to clarify the role of these factors in early response after vaccination. Activation of CLEC5A+ monocytes occurred five days after vaccination (DAV). Following, seven DAV data showed activation of CD4+ and CD8+T cells together with early positive correlations between type II IFN and genes of innate antiviral response (STAT1, STAT2, IRF7, IRF9, OAS1, and RNASEL) as well as antibody levels. Furthermore, individuals with genotypes rs2430561 AT/AA, rs2069718 AG/AA (IFNG), and rs13237944 AC/AA (CLEC5A), exhibited higher expression of IFNG and CLEC5A, respectively. Together, we demonstrated that early IFN-γ and CLEC5A responses, associated with rs2430561, rs2069718, and rs13237944 genotypes, may be key mechanisms in the long-lasting immunity elicited by YF17DD.
Keywords: CLEC5A; interferon gamma; yellow fever vaccine.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

