The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors
- PMID: 33445874
- PMCID: PMC7883342
- DOI: 10.1021/acs.chemrev.0c00779
The Use of Alternative Strategies for Enhanced Nanoparticle Delivery to Solid Tumors
Abstract
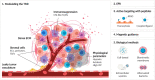
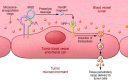
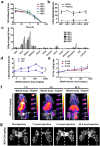
Nanomaterial (NM) delivery to solid tumors has been the focus of intense research for over a decade. Classically, scientists have tried to improve NM delivery by employing passive or active targeting strategies, making use of the so-called enhanced permeability and retention (EPR) effect. This phenomenon is made possible due to the leaky tumor vasculature through which NMs can leave the bloodstream, traverse through the gaps in the endothelial lining of the vessels, and enter the tumor. Recent studies have shown that despite many efforts to employ the EPR effect, this process remains very poor. Furthermore, the role of the EPR effect has been called into question, where it has been suggested that NMs enter the tumor via active mechanisms and not through the endothelial gaps. In this review, we provide a short overview of the EPR and mechanisms to enhance it, after which we focus on alternative delivery strategies that do not solely rely on EPR in itself but can offer interesting pharmacological, physical, and biological solutions for enhanced delivery. We discuss the strengths and shortcomings of these different strategies and suggest combinatorial approaches as the ideal path forward.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









Similar articles
-
Strategies to enhance drug delivery to solid tumors by harnessing the EPR effects and alternative targeting mechanisms.Adv Drug Deliv Rev. 2022 Sep;188:114449. doi: 10.1016/j.addr.2022.114449. Epub 2022 Jul 11. Adv Drug Deliv Rev. 2022. PMID: 35835353 Review.
-
Human solid tumors and clinical relevance of the enhanced permeation and retention effect: a 'golden gate' for nanomedicine in preclinical studies?Nanomedicine (Lond). 2023 Jan;18(2):169-190. doi: 10.2217/nnm-2022-0257. Epub 2023 Apr 12. Nanomedicine (Lond). 2023. PMID: 37042320 Review.
-
Micro/nano-bubble-assisted ultrasound to enhance the EPR effect and potential theranostic applications.Theranostics. 2020 Jan 1;10(2):462-483. doi: 10.7150/thno.37593. eCollection 2020. Theranostics. 2020. PMID: 31903132 Free PMC article. Review.
-
Beyond the EPR effect: Intravital microscopy analysis of nanoparticle drug delivery to tumors.Adv Drug Deliv Rev. 2025 Apr;219:115550. doi: 10.1016/j.addr.2025.115550. Epub 2025 Feb 26. Adv Drug Deliv Rev. 2025. PMID: 40021012 Review.
-
Targeting endothelial permeability in the EPR effect.J Control Release. 2023 Sep;361:212-235. doi: 10.1016/j.jconrel.2023.07.039. Epub 2023 Aug 8. J Control Release. 2023. PMID: 37517543 Review.
Cited by
-
Platinum nanoparticles-embedded single-walled carbon nanotubes as a new carrier for curcumin delivery and investigating its anticancer effect on cell line 4T1.Heliyon. 2024 Jun 26;10(13):e33703. doi: 10.1016/j.heliyon.2024.e33703. eCollection 2024 Jul 15. Heliyon. 2024. PMID: 39027555 Free PMC article.
-
Selenium-integrated conjugated oligomer nanoparticles with high photothermal conversion efficiency for NIR-II imaging-guided cancer phototheranostics in vivo.J Nanobiotechnology. 2023 Sep 4;21(1):314. doi: 10.1186/s12951-023-02080-1. J Nanobiotechnology. 2023. PMID: 37667389 Free PMC article.
-
Implantable, 3D-Printed Alginate Scaffolds with Bismuth Sulfide Nanoparticles for the Treatment of Local Breast Cancer via Enhanced Radiotherapy.ACS Appl Mater Interfaces. 2024 Apr 3;16(13):15718-15729. doi: 10.1021/acsami.3c17024. Epub 2024 Mar 20. ACS Appl Mater Interfaces. 2024. PMID: 38506616 Free PMC article.
-
Smart responsive Fe/Mn nanovaccine triggers liver cancer immunotherapy via pyroptosis and pyroptosis-boosted cGAS-STING activation.J Nanobiotechnology. 2024 Mar 6;22(1):95. doi: 10.1186/s12951-024-02354-2. J Nanobiotechnology. 2024. PMID: 38448959 Free PMC article.
-
Immunomodulatory Prodrug Micelles Imitate Mild Heat Effects to Reshape Tumor Microenvironment for Enhanced Cancer Immunotherapy.ACS Nano. 2024 Feb 20;18(7):5632-5646. doi: 10.1021/acsnano.3c11186. Epub 2024 Feb 12. ACS Nano. 2024. PMID: 38344992 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

