Protocol-Specific Effects of Intermittent Hypoxia Pre-Conditioning on Phrenic Motor Plasticity in Rats with Chronic Cervical Spinal Cord Injury
- PMID: 33446048
- PMCID: PMC8182475
- DOI: 10.1089/neu.2020.7324
Protocol-Specific Effects of Intermittent Hypoxia Pre-Conditioning on Phrenic Motor Plasticity in Rats with Chronic Cervical Spinal Cord Injury
Abstract
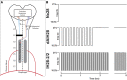
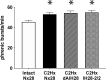
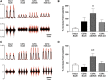
"Low-dose" acute intermittent hypoxia (AIH; 3-15 episodes/day) is emerging as a promising therapeutic strategy to improve motor function after incomplete cervical spinal cord injury (cSCI). Conversely, chronic "high-dose" intermittent hypoxia (CIH; > 80-100 episodes/day) elicits multi-system pathology and is a hallmark of sleep apnea, a condition highly prevalent in individuals with cSCI. Whereas daily AIH (dAIH) enhances phrenic motor plasticity in intact rats, it is abolished by CIH. However, there have been no direct comparisons of prolonged dAIH versus CIH on phrenic motor outcomes after chronic cSCI. Thus, phrenic nerve activity and AIH-induced phrenic long-term facilitation (pLTF) were assessed in anesthetized rats. Experimental groups included: 1) intact rats exposed to 28 days of normoxia (Nx28; 21% O2; 8 h/day), and three groups with chronic C2 hemisection (C2Hx) exposed to either: 2) Nx28; 3) dAIH (dAIH28; 10, 5-min episodes of 10.5% O2/day; 5-min intervals); or 4) CIH (IH28-2/2; 2-min episodes; 2-min intervals; 8 h/day). Baseline ipsilateral phrenic nerve activity was reduced in injured versus intact rats but unaffected by dAIH28 or IH28-2/2. There were no group differences in contralateral phrenic activity. pLTF was enhanced bilaterally by dAIH28 versus Nx28 but unaffected by IH28-2/2. Whereas dAIH28 enhanced pLTF after cSCI, it did not improve baseline phrenic output. In contrast, unlike shorter protocols in intact rats, CIH28-2/2 did not abolish pLTF in chronic C2Hx. Mechanisms of differential responses to dAIH versus CIH are not yet known, particularly in the context of cSCI. Further, it remains unclear whether enhanced phrenic motor plasticity can improve breathing after cSCI.
Keywords: acute intermittent hypoxia; cervical spinal cord injury; chronic intermittent hypoxia; phrenic long-term facilitation; respiratory rehabilitation; sleep apnea.
Conflict of interest statement
No competing financial interests exist.
Figures


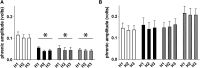
References
-
- van den Berg, M.E., Castellote, J.M., de Pedro-Cuesta, J., and Mahillo-Fernandez, I. (2010). Survival after spinal cord injury: a systematic review. J. Neurotrauma 27, 1517–1528 - PubMed
-
- Winslow, C. and Rozovsky, J. (2003). Effect of spinal cord injury on the respiratory system. Am. J. Phys. Med. Rehabil. 82, 803–814 - PubMed
-
- Winslow, C., Bode, R.K., Felton, D., Chen, D., and Meyer, P.R. (2002). Impact of respiratory complications on length of stay and hospital costs in acute cervical spine injury. Chest 121, 1548–1554 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

